In this study, we demonstrated that inhibition of PI3Ks promoted nocodazole-induced mitotic cell death and reduced mitotic slippage. This finding suggests that using PI3k inhibitors in combination with anti-mitotic drugs may improve cancer treatment outcomes. In summary, the current study demonstrated that the inhibition of PI3K pathway induced mitotic arrest and mitotic cell death and promoted nocodazole-induced mitotic cell death while reducing the occurrence of mitotic slippage. These results suggest a novel role for the PI3K pathway in regulating cell cycle progression during mitosis and preventing mitotic cell death, and provide justification for the use of PI3K inhibitors in combination with anti-mitotic drugs to combat cancer. Isoprenoids constitute one of the largest groups of natural product compounds. They are structurally diverse and include cannabinoids, essential oils, sterols, the prenyl groups of chlorophyll and RNA among others. Isoprenoids are involved in respiration, hormone-based signalling, the post-translational processes that control lipid biosynthesis, meiosis, apoptosis, glycoprotein biosynthesis, and protein degradation. Furthermore, they represent important structural components of cell membranes. All isoprenoids are synthesised from two simple precursors, isopentenyl pyrophosphate and dimethylallyl pyrophosphate. The precursors are provided by two distinct biosynthetic pathways, which are distributed in an organism specific manner. In mammals, the plant cytosol, certain bacteria and trypanosomatids, these compounds are products of the mevalonate pathway. In most eubacteria, algae, chloroplasts, cyanobacteria and apicomplexan parasites the deoxy-xylulose phosphate pathway generates IPP and DMAPP. This biosynthetic route to isoprenoid precursors is an essential aspect of metabolism and the DOXP pathway is a genetically validated target for broad-spectrum antimicrobial drugs against malaria, tuberculosis, and a range of sexually transmitted conditions. The absence of this pathway in humans makes it a particular attractive target for antimicrobial drug discovery. Chemical validation is provided by the anti-malarial compound fosmidomycin, which inhibits 1-deoxy-D-xylulose 5-phosphate reductoisomerase. Two types of IspE inhibitors are known. The majority of IspE inhibitors mimic either the cytidine or phosphate-sugar moiety of the substrate CDP-ME. Crystal structures of AaIspE in complex with cytidine analogues containing a benzimidazole moiety attached to the ribose have been determined suggesting that interactions in the cytidine Y-27632 dihydrochloride ROCK inhibitor pocket are key for binding affinity. Considering the size of these molecules they are rather weak ligands for BU 4061T EcIspE with affinities in the double-digit micromolar range. In contrast, the smaller cytosine analogues bind more tightly to EcIspE with some compounds of this series displaying IC50 values in the low micromolar range. Very recently, non-substrate like EcIspE inhibitors have been reported. The best characterized compounds also have IC50 values in the low micromolar range. They were proposed to bind into the substrate binding site forming stacking interactions with Tyr25 and Phe185; however, the derived structure-activity relationships were not always consistent with this binding mode as large changes to the presumably pistacking moieties did not lead to large changes in affinity. Motivated by the potential of IspE as a target for broadspectrum antimicrobial drugs we sought to discover non-substrate like IspE inhibitors that can 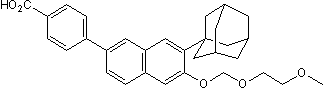 serve as starting points for the development of new antimicrobials. There are several methods for hit discovery.
serve as starting points for the development of new antimicrobials. There are several methods for hit discovery.
Monthly Archives: August 2019
Using both approaches either lead-like or fragment-like libraries can be screened remaining compounds
Lead-like libraries typically deliver fewer but more potent hits compared to screening smaller, fragment-like compounds which often leads to a higher hit rate albeit frequently associated with weaker binding. If the structure of the target is known, molecular docking is a viable in silico method. There are several studies that compare the outcomes of docking and in vitro high-throughput screening. These studies suggest that often the two methods identify different hit compounds. Reasons for this are that as a result of virtual screening usually only few compounds are tested experimentally which allows more robust assays to be used and testing at higher concentrations which can identify weaker inhibitors. Further, much larger libraries can be screened computationally than it is affordable to screen biochemically. On the other hand, due to shortcomings in docking algorithms and scoring functions, potential hits might be missed when only relying on computational methods. To benefit from the advantageous of these complementary strategies, we decided to apply both for hit discovery for IspE. The substrate and co-factor binding sites of IspE are highly conserved across difference species.. Therefore, in principle, given the high level of conservation in IspE across species either structure could serve as 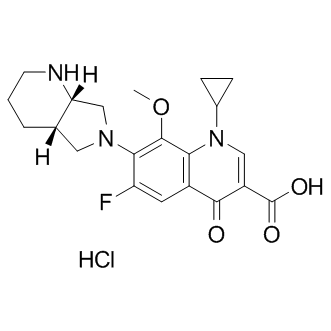 a template for structurebased design of inhibitors with broad-spectrum antimicrobial activity. However, since we had been able to reproducibly crystallize and gain most crystallographic information with BKM120 AaIspE we decided to use the former for virtual screening. The intention was then to determine crystal structures of new inhibitors in complex with AaIspE. As A. aeolicus is a thermophilic organism with the optimal temperature of AaIspE activity near 60uC and working at such elevated temperatures is not practical for a biochemical screen, it was decided to use E. coli IspE for ligand binding characterisation. The high level of sequence conservation provided confidence in this approach. Here, we report on our hit discovery efforts for IspE. The crystal structures were exploited for a structure-based ligand design approach leading to efficiently binding fragments likely addressing the cytidine-binding site. In addition, a biochemical screen of a focussed compound library was carried out resulting in two inhibitors with binding affinities in the low micromolar range. Hit compounds from both approaches were expanded to compound series. Compounds of these series have high ligand efficiencies and possess favourable physico-chemical Cabozantinib purchase properties representing promising starting points for the synthesis of new IspE inhibitors. In addition, we compared the performance of in silico and in vitro screening and discuss their strengths and weaknesses. Analysis of AaIspE crystal structures suggested that the cytidine moiety plays a key role in ligand binding. The cytidine binding site is formed by two aromatic amino acids which form stacking and edge-face interactions with the cytidine ring and a histidine residue that stabilizes ligand binding by forming hydrogen bonds with N3 and the exocyclic carbonyl and amino groups. This narrow cleft, promoting aromatic and polar interactions, appears well suited to accommodate small compounds based on scaffolds distinct from cytidine with potential to display high ligand efficiency. A hierarchical screening strategy was adopted to retrieve fragments binding into the cytidine pocket of IspE. First, a database of commercially available compounds was filtered according to physico-chemical criteria. Next, a pharmacophore hypothesis was derived and used to screen all compounds passing the first filter step.
a template for structurebased design of inhibitors with broad-spectrum antimicrobial activity. However, since we had been able to reproducibly crystallize and gain most crystallographic information with BKM120 AaIspE we decided to use the former for virtual screening. The intention was then to determine crystal structures of new inhibitors in complex with AaIspE. As A. aeolicus is a thermophilic organism with the optimal temperature of AaIspE activity near 60uC and working at such elevated temperatures is not practical for a biochemical screen, it was decided to use E. coli IspE for ligand binding characterisation. The high level of sequence conservation provided confidence in this approach. Here, we report on our hit discovery efforts for IspE. The crystal structures were exploited for a structure-based ligand design approach leading to efficiently binding fragments likely addressing the cytidine-binding site. In addition, a biochemical screen of a focussed compound library was carried out resulting in two inhibitors with binding affinities in the low micromolar range. Hit compounds from both approaches were expanded to compound series. Compounds of these series have high ligand efficiencies and possess favourable physico-chemical Cabozantinib purchase properties representing promising starting points for the synthesis of new IspE inhibitors. In addition, we compared the performance of in silico and in vitro screening and discuss their strengths and weaknesses. Analysis of AaIspE crystal structures suggested that the cytidine moiety plays a key role in ligand binding. The cytidine binding site is formed by two aromatic amino acids which form stacking and edge-face interactions with the cytidine ring and a histidine residue that stabilizes ligand binding by forming hydrogen bonds with N3 and the exocyclic carbonyl and amino groups. This narrow cleft, promoting aromatic and polar interactions, appears well suited to accommodate small compounds based on scaffolds distinct from cytidine with potential to display high ligand efficiency. A hierarchical screening strategy was adopted to retrieve fragments binding into the cytidine pocket of IspE. First, a database of commercially available compounds was filtered according to physico-chemical criteria. Next, a pharmacophore hypothesis was derived and used to screen all compounds passing the first filter step.
Proceeding from successful transgenic constructed chemical library of analogs for inhibitors of Ab oligomerization
This library includes compounds with variations on carbon spacer length between phenolic rings, a variety of ring substitutions, as well as substitutions to the central methylene carbon of curcumin. In general, our BKM120 studies indicate that at least one enone group on the spacer is necessary for measureable anti- Ab aggregation activity. The most striking SB431542 feature among compounds in both the 7- and 5-carbon series listed in Figure 1 is the presence of an a/bunsaturated carbon spacer. None of the compounds with saturated spacers demonstrated inhibitory activity, indicating that an unsaturated spacer between aryl rings is essential for anti- Ab aggregation activity. A similar finding was reported by Begum, et al., when they compared the antiamyloidogenic activities of dietary curcumin with that of tetrahydrocurcumin. Further study of Figure 1 reveals novel structure/function relationships with regard to specific substitutions to the aryl rings. Ortho-substitutions do not appear to contribute to improved inhibitor activity; however, maintaining methoxyl and hydroxyl substitutions in the meta- and parapositions on the aryl rings is necessary for comparable or improved inhibitory activity when measured against curcumin. In the 5carbon series, one compound was significantly improved over that of curcumin, compound 8, which has hydroxyl groups in both meta- and para-positions of the aryl 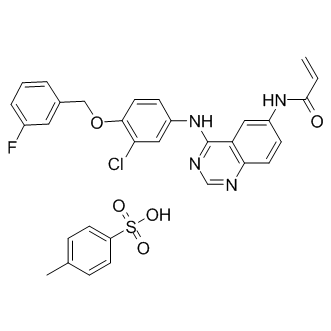 rings. The most improved inhibitors identified in the 7-carbon series have their meta- and para-substituted methoxyl and hydroxyl groups reversed from that of curcumin, as with compound 1, or methoxyl groups placed in both positions, as with compound 2. The simple substitution of the para-hydroxy group on curcumin with a methoxy substitution improved inhibitor function by 6-7-fold over that measured for curcumin, making compound 2 our most potent lead analog for anti-Ab aggregation activity. Additional challenges lie ahead to improve the bioactivity of our curcumin-derived analog in order to increase the therapeutic dose to the CNS. Questions in regard to bioavailability have plagued the use of curcumin as a potential therapeutic for a number of years. Clinical trials have shown that the inherent bioavailability of orally administered curcumin is relatively low when factoring in intestinal absorption, liver metabolism and BBB penetrance. However, in spite of these difficulties, dietary supplementation of curcumin administered to aged APPtransgenic mice significantly lowered Ab deposition in the CNS. These findings clearly show that curcumin is able to enter the circulation and cross the BBB in sufficient quantities to reduce amyloid burden. To improve upon this property, we anticipate that the methoxy substitution on our lead compound 2 will decrease polarity and increase lipid membrane solubility thereby improving passive diffusion across the blood brain barrier and access to the CNS. Similar observations have been made for other inhibitors of Ab aggregation such as Chrysamine G. In this study, the more lipophilic compound Chrysamine G was compared with Congo Red and found to readily cross the BBB in normal mice, achieving a brain:blood ratio of greater than 10:1. Moreover, metabolic inactivation poses other challenges to maintaining bioactivity. In this respect, the hydroxyl groups on curcumin are modified by enzymes found in the liver, kidney and intestinal mucosa to form curcumin glucuronides and curcumin sulfates. The methoxy substitution for these hydroxyl groups on our lead compound 2 should prevent these glucuronide and sulfate additions and contribute to sustained bioactivity.
rings. The most improved inhibitors identified in the 7-carbon series have their meta- and para-substituted methoxyl and hydroxyl groups reversed from that of curcumin, as with compound 1, or methoxyl groups placed in both positions, as with compound 2. The simple substitution of the para-hydroxy group on curcumin with a methoxy substitution improved inhibitor function by 6-7-fold over that measured for curcumin, making compound 2 our most potent lead analog for anti-Ab aggregation activity. Additional challenges lie ahead to improve the bioactivity of our curcumin-derived analog in order to increase the therapeutic dose to the CNS. Questions in regard to bioavailability have plagued the use of curcumin as a potential therapeutic for a number of years. Clinical trials have shown that the inherent bioavailability of orally administered curcumin is relatively low when factoring in intestinal absorption, liver metabolism and BBB penetrance. However, in spite of these difficulties, dietary supplementation of curcumin administered to aged APPtransgenic mice significantly lowered Ab deposition in the CNS. These findings clearly show that curcumin is able to enter the circulation and cross the BBB in sufficient quantities to reduce amyloid burden. To improve upon this property, we anticipate that the methoxy substitution on our lead compound 2 will decrease polarity and increase lipid membrane solubility thereby improving passive diffusion across the blood brain barrier and access to the CNS. Similar observations have been made for other inhibitors of Ab aggregation such as Chrysamine G. In this study, the more lipophilic compound Chrysamine G was compared with Congo Red and found to readily cross the BBB in normal mice, achieving a brain:blood ratio of greater than 10:1. Moreover, metabolic inactivation poses other challenges to maintaining bioactivity. In this respect, the hydroxyl groups on curcumin are modified by enzymes found in the liver, kidney and intestinal mucosa to form curcumin glucuronides and curcumin sulfates. The methoxy substitution for these hydroxyl groups on our lead compound 2 should prevent these glucuronide and sulfate additions and contribute to sustained bioactivity.
The activities of which are influenced by local concentrations of metabolites or the small molecule c46 cocrystallized
We identified a structurally diverse set of small scaffolds that may be overlaid onto distinct regions of the c64 ligand as present in the crystal structure, and our M. tuberculosis H37Rv active sets were searched for hits that contain these substructures. Twelve such 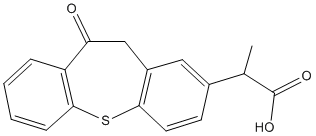 small scaffolds led to the ASP1517 clinical trial identification of forty-three compounds among the reported TB actives, and these are shown in Figure S1. Out of the M. tuberculosis H37Rv actives identified based on these searches thirty-three compounds were selected for evaluation against Mt-GuaB2. Compound 7759844 was docked into the Mt-GuaB2-IMP complex structure using the InducedFit docking protocol from the Schrodinger software package that treated residues within 5 A ? of docked ligand conformers as flexible. Two possible docked orientations were predicted for this ligand, one showed excellent consistency with the limited SAR data available for this lead scaffold, as listed in Table 3. Therefore, we considered this mode as our working model of 7759844 binding to Mt-GuaB2, for further validation. The potential of IMPDH for immunosuppressive, cancer and antiviral chemotherapy has been previously explored. Recently, Mt-GuaB2 has gained attention as a promising anti-mycobacterial target, associated with a number of distinct inhibitor scaffolds. The applicability of available bacterial IMPDH inhibitors may be limited by toxicity issues as there is an important human homolog, and lead optimization must focus on selectivity as well as potency. In the search for new lead compounds as potential MtGuaB2 inhibitors, we employed a BU 4061T Proteasome inhibitor designed scaffold-based approach utilizing the IMPDH crystal structure with c64 cocrystallized in the active site. M. tuberculosis H37Rv active sets from our previous screens were searched based on a set of designed scaffolds listed in Figure S1 as well as a known IMPDH scaffold. Of the identified M. tuberculosis actives, we evaluated thirty-three compounds for their potential to inhibit Mt-GuaB2 functional activity and for their inhibitory potency against M. smegmatis. In the present study, the assay was adapted to a 200 ml volume, conditions were optimized by varying concentrations of the substrate IMP, the cofactor NAD+and the enzyme, and the Km were determined for IMP. The optimized assay yielded a reaction that proceeded linearly over a 5 min period. Next, we studied the inhibition of Mt-GuaB2 by a series of commercially available compounds, primarily from the Chembridge and Life Chemicals libraries. When compared to the DPU’s, these compounds show an improvement in enzyme inhibitory activity, yielding low micromolar inhibitors. The Ki values were obtained for all inhibitors with respect to both substrates IMP and the patterns of inhibition were inferred from the graphs. Among the Chembridge compounds, four exhibited low micromolar affinity Ki values. Out of the Life Chemicals compound set, F1374-1083 showed a low micromolar inhibition constant. All inhibitors from the Chembridge compound set showed an uncompetitive pattern of inhibition against both substrates. Uncompetitive inhibition has been observed previously versus both IMP and NAD +, in the case of compounds having a strong preference for the E-XMP* complex. The observation of an uncompetitive pattern of inhibition also depended on assay conditions. In the case of uncompetitive inhibition, both the substrates bind to the enzyme before the inhibitor binds. The uncompetitive inhibitors have an advantage as drugs since inhibition increased due to accumulation of substrates. The post-translational modification of core histones plays a central role in epigenetic gene regulation. Modifications are put in place by families of modifying and demodifying enzymes.
small scaffolds led to the ASP1517 clinical trial identification of forty-three compounds among the reported TB actives, and these are shown in Figure S1. Out of the M. tuberculosis H37Rv actives identified based on these searches thirty-three compounds were selected for evaluation against Mt-GuaB2. Compound 7759844 was docked into the Mt-GuaB2-IMP complex structure using the InducedFit docking protocol from the Schrodinger software package that treated residues within 5 A ? of docked ligand conformers as flexible. Two possible docked orientations were predicted for this ligand, one showed excellent consistency with the limited SAR data available for this lead scaffold, as listed in Table 3. Therefore, we considered this mode as our working model of 7759844 binding to Mt-GuaB2, for further validation. The potential of IMPDH for immunosuppressive, cancer and antiviral chemotherapy has been previously explored. Recently, Mt-GuaB2 has gained attention as a promising anti-mycobacterial target, associated with a number of distinct inhibitor scaffolds. The applicability of available bacterial IMPDH inhibitors may be limited by toxicity issues as there is an important human homolog, and lead optimization must focus on selectivity as well as potency. In the search for new lead compounds as potential MtGuaB2 inhibitors, we employed a BU 4061T Proteasome inhibitor designed scaffold-based approach utilizing the IMPDH crystal structure with c64 cocrystallized in the active site. M. tuberculosis H37Rv active sets from our previous screens were searched based on a set of designed scaffolds listed in Figure S1 as well as a known IMPDH scaffold. Of the identified M. tuberculosis actives, we evaluated thirty-three compounds for their potential to inhibit Mt-GuaB2 functional activity and for their inhibitory potency against M. smegmatis. In the present study, the assay was adapted to a 200 ml volume, conditions were optimized by varying concentrations of the substrate IMP, the cofactor NAD+and the enzyme, and the Km were determined for IMP. The optimized assay yielded a reaction that proceeded linearly over a 5 min period. Next, we studied the inhibition of Mt-GuaB2 by a series of commercially available compounds, primarily from the Chembridge and Life Chemicals libraries. When compared to the DPU’s, these compounds show an improvement in enzyme inhibitory activity, yielding low micromolar inhibitors. The Ki values were obtained for all inhibitors with respect to both substrates IMP and the patterns of inhibition were inferred from the graphs. Among the Chembridge compounds, four exhibited low micromolar affinity Ki values. Out of the Life Chemicals compound set, F1374-1083 showed a low micromolar inhibition constant. All inhibitors from the Chembridge compound set showed an uncompetitive pattern of inhibition against both substrates. Uncompetitive inhibition has been observed previously versus both IMP and NAD +, in the case of compounds having a strong preference for the E-XMP* complex. The observation of an uncompetitive pattern of inhibition also depended on assay conditions. In the case of uncompetitive inhibition, both the substrates bind to the enzyme before the inhibitor binds. The uncompetitive inhibitors have an advantage as drugs since inhibition increased due to accumulation of substrates. The post-translational modification of core histones plays a central role in epigenetic gene regulation. Modifications are put in place by families of modifying and demodifying enzymes.
Importantly the tool has a dedicated purpose in HTS assay design and only requires basic knowledge in enzyme kinetics
However, conditions such as those commonly applied to HTS for enzyme inhibitors often violate this approximation and make interpretations based on the MM equation for initial reaction velocity less reliable. Furthermore, such assays are often associated with a high enough level of substrate turnover to render the phenomenon of product inhibition significant, thus complicating interpretation of observed  inhibition further. Consequently, interpretation of data from experiments such as HTS, as well as the design of HTS assay conditions, should ideally be founded on progress curve analysis. Since the MM rate law cannot be analytically integrated to explicitly express product concentration as a function of time and in terms of kcat and Km, this has to be achieved by numeric approaches. Due to these issues, a tool in spreadsheet format specifically designed to simplify the analysis and design of HTS assays has been developed. The tool is simple to use and only requires knowledge in standard enzyme kinetics. It provides comparative analysis of the progress of uninhibited versus inhibited reactions for common inhibitory mechanisms and takes reaction reversibility and enzyme half-life into account. Reactions are simulated in response to adjustment of kinetic parameters and key data are automatically deduced. Furthermore, as for any method relying on progress curve analysis, the linearity and range of the response must be accounted for when interpreting and comparing experimental data with simulated data. To further evaluate the validity of the simulations, progress curves were also collected for another enzyme system, peptidolysis by presequence peptidase, with and without bestatin as an inhibitor. Kinetic parameters were derived by leastsquares model fitting. As a comparison, an experiment to determine kinetic parameters with initial reaction rates at increasing substrate concentrations was also performed. Curve fitting gave a good data-to-model agreement, for both progress curves and the initial reaction rate experiment, and kinetic parameters derived with the two methods were very similar. A tool for the simulation and comparative analysis of enzymatic progress curves for common types of inhibition has been developed. The tool can be downloaded as supplemental material or obtained from the author. The tool provides AbMole BioScience kinase inhibitors accurate simulation of experimental progress curves – given that the enzyme system under study can be approximated by the underlying model, as in any simulation approach. Reaction parameters and concentrations can be adjusted to directly R428 1037624-75-1 observe the effects on displayed progress curves and essential data are deduced and clearly presented. The tool is particularly intended to support experimental design and to facilitate interpretation of data obtained in end-point assays in HTS for enzyme inhibitors. In these processes the tool can be used to: study the effect of reaction conditions on the choice of observation window, tune reaction condition in favor of a particular type of inhibition, investigate the amount of substrate turn-over that can be allowed to increase the assay signal without severely affecting observed inhibition, adapt assay conditions to an enzyme with pronounced product inhibition, or to guide the selection of hit cut-off criteria while accounting for product inhibition, reaction reversibility, and substrate turn-over.
inhibition further. Consequently, interpretation of data from experiments such as HTS, as well as the design of HTS assay conditions, should ideally be founded on progress curve analysis. Since the MM rate law cannot be analytically integrated to explicitly express product concentration as a function of time and in terms of kcat and Km, this has to be achieved by numeric approaches. Due to these issues, a tool in spreadsheet format specifically designed to simplify the analysis and design of HTS assays has been developed. The tool is simple to use and only requires knowledge in standard enzyme kinetics. It provides comparative analysis of the progress of uninhibited versus inhibited reactions for common inhibitory mechanisms and takes reaction reversibility and enzyme half-life into account. Reactions are simulated in response to adjustment of kinetic parameters and key data are automatically deduced. Furthermore, as for any method relying on progress curve analysis, the linearity and range of the response must be accounted for when interpreting and comparing experimental data with simulated data. To further evaluate the validity of the simulations, progress curves were also collected for another enzyme system, peptidolysis by presequence peptidase, with and without bestatin as an inhibitor. Kinetic parameters were derived by leastsquares model fitting. As a comparison, an experiment to determine kinetic parameters with initial reaction rates at increasing substrate concentrations was also performed. Curve fitting gave a good data-to-model agreement, for both progress curves and the initial reaction rate experiment, and kinetic parameters derived with the two methods were very similar. A tool for the simulation and comparative analysis of enzymatic progress curves for common types of inhibition has been developed. The tool can be downloaded as supplemental material or obtained from the author. The tool provides AbMole BioScience kinase inhibitors accurate simulation of experimental progress curves – given that the enzyme system under study can be approximated by the underlying model, as in any simulation approach. Reaction parameters and concentrations can be adjusted to directly R428 1037624-75-1 observe the effects on displayed progress curves and essential data are deduced and clearly presented. The tool is particularly intended to support experimental design and to facilitate interpretation of data obtained in end-point assays in HTS for enzyme inhibitors. In these processes the tool can be used to: study the effect of reaction conditions on the choice of observation window, tune reaction condition in favor of a particular type of inhibition, investigate the amount of substrate turn-over that can be allowed to increase the assay signal without severely affecting observed inhibition, adapt assay conditions to an enzyme with pronounced product inhibition, or to guide the selection of hit cut-off criteria while accounting for product inhibition, reaction reversibility, and substrate turn-over.