Although confirmation of the efficacy of this pharmacological approach awaits completion of large clinical trials, the adjuvant use of these compounds is common in patients that do not meet targeted reductions of lipoproteins while taking statins. Given the high prevalence of lipid metabolism disorders it is desirable to identify lead compounds that can be developed into new drugs that inhibit lipid absorption via novel mechanisms. Here we report the utility of using the zebrafish for this purpose. Because of their small size, optical transparency zebrafish larvae are well suited for chemical library screens using fluorescent, histochemical or morphological assays. Indeed, a great advantage of chemical screens in zebrafish is the ability to rapidly assess compound efficacy and toxicity in vivo. Given the high degree of conservation of lipid metabolism in teleost fish and mammals, it is likely that compounds identified in a zebrafish screen will act through comparable mechanisms in mammals. Here we report the results of a pilot screen of a non-biased chemical library through which we identified 7 novel compounds that inhibited the absorption of fluorescent lipid analogues. We show that compounds identified in the primary screening assay can be rapidly prioritized for testing in mammals using a variety of simple, yet Everolimus highly informative in vivo secondary assays. The secondary assays also Torin 1 company provided insights into the compounds�� mechanism of action, which could be 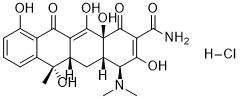 distinguished from the effects of orlistat and ezetimibe in zebrafish larvae. Surprisingly, we found that ezetimibe inhibited absorption of not only cholesterol analog, but also long chain fatty acid and phopholipid analogs. Together, these findings demonstrate the feasibility of conducting screens for compounds that interfere with complex physiological processes using the zebrafish. The screening assays used for this study were derived from previous work using fluorescent lipid reporters in zebrafish larvae. Following their ingestion, the fluorescent metabolites of these reporters are first detected in the gallbladder of live larvae and later the intestinal lumen following gallbladder contraction. The compounds are used at low concentrations and they are rapidly absorbed from the intestinal lumen, thus their fluorescence emission is not detected in the intestinal lumen immediately after ingestion or when absorption in inhibited. Fluorescence emission from one of the analogues, the phospholipid PED-6, is quenched prior to metabolism by luminal phospholipase. Thin layer chromatographic analyses of bile from adult fish, or total body lipids of 5 dpf larvae, showed that PED-6, which is labeled with a BODIPY labeled short chain fatty acid at the sn-2 position, is metabolized to cholesterol esters, phospholipids and possibly, triglyceride. Free PED6 was not detected in either assay. For the primary screen, 5 day post-fertilization larvae were arrayed in 96 well plates and soaked overnight in test compounds. The following morning larvae were soaked in PED-6 for 6 hours after which a qualitative visual assessment of gallbladder fluorescence was made using an inverted compound microscope. Reduced gallbladder fluorescence, the endpoint we use to identify active compounds in the primary screen, could not differentiate compounds that inhibited lipid absorption from those that interfered with swallowing, phospholipase activity or hepatic metabolism and biliary secretion. As described below, secondary assays were devised to distinguish these mechanistic possibilities.
distinguished from the effects of orlistat and ezetimibe in zebrafish larvae. Surprisingly, we found that ezetimibe inhibited absorption of not only cholesterol analog, but also long chain fatty acid and phopholipid analogs. Together, these findings demonstrate the feasibility of conducting screens for compounds that interfere with complex physiological processes using the zebrafish. The screening assays used for this study were derived from previous work using fluorescent lipid reporters in zebrafish larvae. Following their ingestion, the fluorescent metabolites of these reporters are first detected in the gallbladder of live larvae and later the intestinal lumen following gallbladder contraction. The compounds are used at low concentrations and they are rapidly absorbed from the intestinal lumen, thus their fluorescence emission is not detected in the intestinal lumen immediately after ingestion or when absorption in inhibited. Fluorescence emission from one of the analogues, the phospholipid PED-6, is quenched prior to metabolism by luminal phospholipase. Thin layer chromatographic analyses of bile from adult fish, or total body lipids of 5 dpf larvae, showed that PED-6, which is labeled with a BODIPY labeled short chain fatty acid at the sn-2 position, is metabolized to cholesterol esters, phospholipids and possibly, triglyceride. Free PED6 was not detected in either assay. For the primary screen, 5 day post-fertilization larvae were arrayed in 96 well plates and soaked overnight in test compounds. The following morning larvae were soaked in PED-6 for 6 hours after which a qualitative visual assessment of gallbladder fluorescence was made using an inverted compound microscope. Reduced gallbladder fluorescence, the endpoint we use to identify active compounds in the primary screen, could not differentiate compounds that inhibited lipid absorption from those that interfered with swallowing, phospholipase activity or hepatic metabolism and biliary secretion. As described below, secondary assays were devised to distinguish these mechanistic possibilities.
Monthly Archives: August 2019
As well as its ternary complex of three structurally dissimilar aminoglycosides are known
Perhaps the most different among the APHs examined structurally is APH-Ia -IIIa). APH-Ia is an atypical APH which phosphorylates only one aminoglycoside, spectinomycin, that is distinct from the other aminoglycoside antibiotics. Its apo, AMP-bound and the ternary structures have been determined, making it the second structurally most studied member of the APH family. Combined, these studies reveal that although members of the APH family share low similarities in sequence and their ligand specificity varies greatly, their overall three-dimensional fold is homologous to each other and to that of ePKs. To further advance the development of APH inhibitors, we describe here the three-dimensional structure of the APH-IIIa and APH-Ia in complex with CKI-7. These inhibitor bound crystal structures of APHs represent the first structures of a eukaryotic protein kinase inhibitor complexed to enzymes that are not eukaryotic protein kinases. Comparison of the inhibitor-bound APH-IIIa and APH-Ia complexes with the nucleotide-bound APH-IIIa and APH-Ia, as well as the CKI-7-bound casein kinase 1 reveals the different inhibitor binding modes as well as topological features that could be exploited in the development of DAPT inhibitors with enhanced affinity and selectivity for APH enzymes. The hydrogen bond between the cyclic nitrogen and a main chain amide in the linker of the enzyme is also present in the CKI-7-bound CK1. The equivalent hydrogen bond observed between N1 of adenine and the tethering segment in the three enzymes is conserved in all adenine-binding to ePKs. This hydrogen bond is not unique to isoquinolinesulfonamide type inhibitors binding to the three enzymes discussed here. A majority of ePK crystal structures complexed with an ATP-competitive inhibitor form at least one hydrogen bond with residues in the linker region, mimicking the ones between N1 and/ or the exocyclic N6 of the adenine and the enzyme. The significance of the hydrogen bond interaction is corroborated by a previous observation in which naphthalene sulfonamide molecules did not display selective inhibition against ePKs until the allcarbon naphthalene ring is substituted with an isoquinoline. In contrast to APH-IIIa in which the aminoethyl tail adopts an extended conformation, this groups adopts the same conformation and is placed in the equivalent area as that in APH-Ia. The aminoethyl tail found in the CK1 structure bends back toward the sulfonyl group and forms an intramolecular interaction between the terminal nitrogen atom and the equatorial sulfonyl oxygen  atom. Deviating slightly from the binding mode of CKI-7 to APH-Ia, the contact between the Nb of the aminoethyl and carbonyl of Leu88 located in the linker of the enzyme is achieved via a water molecule, compared to a direct interaction observed in APH-Ia. Hemostasis is one of the most important processes in organisms, and disorders in this system cause deaths under a variety of pathologies. The activation of blood coagulation can be caused by trauma, sepsis, inflammation, obstetric practice and in the course of XL-184 surgical operations, especially operations using extracorporal blood circulation. Hypercoagulation has also been observed during infusion therapy with large volumes of crystalloid plasma substitutes. Oral contraception and artificial vessels or cardiac valves may be sources of minor but permanent activation of coagulation, eventually exhausting the pool of coagulation inhibitors and giving rise to thrombotic events. Thrombotic pathologies are a result of an imbalance in the activity of thrombin, a key enzyme of the coagulation cascade, and its natural inhibitors.
atom. Deviating slightly from the binding mode of CKI-7 to APH-Ia, the contact between the Nb of the aminoethyl and carbonyl of Leu88 located in the linker of the enzyme is achieved via a water molecule, compared to a direct interaction observed in APH-Ia. Hemostasis is one of the most important processes in organisms, and disorders in this system cause deaths under a variety of pathologies. The activation of blood coagulation can be caused by trauma, sepsis, inflammation, obstetric practice and in the course of XL-184 surgical operations, especially operations using extracorporal blood circulation. Hypercoagulation has also been observed during infusion therapy with large volumes of crystalloid plasma substitutes. Oral contraception and artificial vessels or cardiac valves may be sources of minor but permanent activation of coagulation, eventually exhausting the pool of coagulation inhibitors and giving rise to thrombotic events. Thrombotic pathologies are a result of an imbalance in the activity of thrombin, a key enzyme of the coagulation cascade, and its natural inhibitors.
The poorly conserved disulphides show gaps in their alignment whether PAbN really acts on efflux pumps
We observed that PAbN increased FDG hydrolysis and leakage of fluorescein in all strains, especially in pump deletion mutants, by this new method. Since PAbN was first reported, it has been universally recognized as an efflux pump inhibitor, and the effect of PAbN on MDRP S1 was remarkably synergistic with all the agents examined in this study. In the presence of these antibiotics, the PBPs form a lethal covalent penicilloyl-enzyme complex that blocks the normal transpeptidation reaction; this finally results in bacterial death. However, Gram-negative bacteria have acquired Vorinostat HDAC inhibitor resistance to blactams mainly through three different strategies: production of a specific b-lactam hydrolase; presence of low-affinity PBPs; and active expulsion of b-lactams via efflux pumps. There is thus an urgent need to develop new antibiotics to AG-013736 overcome the challenge of bacterial resistance to existing antimicrobials. The crystal structure of PBP2a in both its apo form and complexed to b-lactams has shown that methicillin resistance is achieved through a distorted active site, which requires an energetically costly b3 strand movement to allow acylation by blactam antibiotics. One of the possibilities to overcome this intrinsic poor acylation efficiency of PBP2a is to design new blactams that have improved binding affinities due to increased noncovalent interactions between the inhibitor and the active site. On the other hand, noncovalent compounds that bind tightly to the active site without acylation might also provide highly effective inhibitors. Noncovalent inhibitors will not require the unfavorable conformational changes in the active site of PBP2a that are required for acylation, and they will hopefully also not be susceptible to b-lactamases. To date, only a few noncovalent inhibitors of PBPs have been described, and so we screened our in-house bank of compounds for potential inhibition of this important drug target. The waning prospect of an effective treatment for bacterial infections due to the emergence and spread of resistance to  antibiotics in pathogens has been exacerbated by the lack of novel antibacterials being introduced to the market. An alternative and parallel approach in supporting the mitigation of the antibiotic resistance problem is to develop adjuvants that could interfere with the mechanism of resistance and hence restore the action of antibiotics. Such a strategy has been effectively employed to combat resistance to b-lactams due to b-lactamase activity. For aminoglycosides, a group of antibiotics used to treat serious nosocomial infections, the main mechanism of resistance is via the enzymatic inactivation of the drug by acetyltransferases, nucleotidyltransferases, or phosphotransferases. This implies that inhibitors of these enzymes could be exploited for the development of drug-adjuvant therapy. Among the three types of aminoglycoside-modifying enzymes, aminoglycoside phosphotransferases or kinases yield the highest levels of resistance thereby providing a rationale for focusing inhibitor development for these specific resistance factors. The investigation of APH inhibitors that target the ATP-binding pocket was facilitated by the structural similarities between the aminoglycoside resistance enzyme APH-IIIa and serine/threonine and tyrosine eukaryotic protein kinases, especially in the Nterminal lobe. It was subsequently shown that APH-IIIa can be inhibited by protein kinase inhibitors of the isoquinolinesulfonamide family and they are competitive with ATPbinding. However, APH-IIIa remains the most extensively studied due to its broad substrate spectrum.
antibiotics in pathogens has been exacerbated by the lack of novel antibacterials being introduced to the market. An alternative and parallel approach in supporting the mitigation of the antibiotic resistance problem is to develop adjuvants that could interfere with the mechanism of resistance and hence restore the action of antibiotics. Such a strategy has been effectively employed to combat resistance to b-lactams due to b-lactamase activity. For aminoglycosides, a group of antibiotics used to treat serious nosocomial infections, the main mechanism of resistance is via the enzymatic inactivation of the drug by acetyltransferases, nucleotidyltransferases, or phosphotransferases. This implies that inhibitors of these enzymes could be exploited for the development of drug-adjuvant therapy. Among the three types of aminoglycoside-modifying enzymes, aminoglycoside phosphotransferases or kinases yield the highest levels of resistance thereby providing a rationale for focusing inhibitor development for these specific resistance factors. The investigation of APH inhibitors that target the ATP-binding pocket was facilitated by the structural similarities between the aminoglycoside resistance enzyme APH-IIIa and serine/threonine and tyrosine eukaryotic protein kinases, especially in the Nterminal lobe. It was subsequently shown that APH-IIIa can be inhibited by protein kinase inhibitors of the isoquinolinesulfonamide family and they are competitive with ATPbinding. However, APH-IIIa remains the most extensively studied due to its broad substrate spectrum.
Lack of auto-regulatory mechanisms suggest that they are mainly regulated by tightly controlled gene expression levels
TCL1-family proteins are 13�C15 kDa non-enzymatic interaction modules. Their restricted physiological expression and apparent. Normal time- and tissue-restricted expression of TCL1 genes is important for efficient fertility, and the development of T and B cell lineages. Deregulation of TCL1 expression can cause the transformation of mature human T and B lymphocytes, and is associated with a variety of human diseases, including VE-821 1232410-49-9 T-cell prolymphocytic leukaemia, B-cell chronic leukaemia, B-cell lymphoma and germ-cell tumours ]. TCL1A is a co-activator of AKT, a serine/threonine protein kinase that regulates many cellular processes, including proliferation and survival. TCL1A forms homodimers, and each protomer binds one pleckstrin homology domain of AKT. Co-activation may thus result from stabilising AKT in an open conformation, from promoting AKT auto-phosphorylation in trans, and/or from reinforcing AKT attachment to the membrane, where its effectors are located. Activated AKT can promote cell survival by phosphorylating downstream targets such as BAD. AKT can also activate the NF-kB transcription factor, possibly by phosphorylating the IkB kinase. Phosphorylated IKK phosphorylates IkB, leading to dissociation of the IkB:NF-kB complex. Liberated NF-kB subsequently translocates to the nucleus to activate survival-inducing genes. However, the effects of TCL1A on AKT are insufficient to fully explain TCL1A oncogenesis. For example, AKT phosphorylation or inactivation kinetics do not consistently relate to TCL1A expression in both transgenic mice and patient�Cderived neoplasia, and AKT activation alone does not necessarily cause tumours in B cells. This suggests that additional effects, and effectors, of TCL1A exist. Recently, it was reported that the NF-kB pathway is important for B-CLL in transgenic mouse models, and that TCL1A activates NF-kB through an AKTindependent route. Corroborating an AKT-independent targeting of NF-kB by TCL1A, we here report that TCL1A binds directly to the NF-kB inhibitor IkB. Several lines of evidence suggest an AKT-independent action of TCL1A on NF-kB pathways. We here sharpened this picture by showing that TCL1A and the NF-kB inhibitor IkB associate in vitro, in yeast-two-hybrid systems, and when transiently overexpressed in 293 cells. We also showed in vitro that TCL1A competed with NF-kB for binding to IkB, suggesting that TCL1A interferes with the inhibitory interaction between IkB and NF-kB. Since TCL1A binds to the same first two ankyrin repeats of IkB which also interact with helices aA and aB of RELA, it is likely that TLC1A and RELA use overlapping binding sites on IkB. However, we can not exclude that IkB binding to one partner causes conformational changes that affect allosterically the binding site of the other partner. On a cellular level, the effect expected to arise from the competition of TCL1A and NF-kB for I kB would be an AKTindependent NF-kB-activation by TCL1A. This was indeed recently observed by Pekarsky et al.. These authors reported that this effect involves the association between TCL1A and p300. The molecular details of the association between TCL1A and p300 remain elusive. Since both proteins are multivalent adaptor proteins, sequential or concomitant interactions of TCL1A with IkB are not excluded, and may be necessary for activation of NF-kB. The description of several AKT-independent TCL1A targets suggests that TCL1A affects a number of alternative and interconnected signalling pathways. Indeed, depending on the cell type and PF-04217903 experimental conditions, both NF-kB activation and inhibition by TCL1A were reported, as well as alternative, NF-kB independent routes. Thus, TCL1A increasingly appears as a polyvalent adaptor protein, whose cellular action is dramatically affected by its sub-cellular concentration  and the availability of potential targets. The affinity we measured between IkB and TCL1A was about 1,000 fold weaker than the one previously reported between IkB and NF-kB.
and the availability of potential targets. The affinity we measured between IkB and TCL1A was about 1,000 fold weaker than the one previously reported between IkB and NF-kB.
Leading to a significant amplification of apoptotic effects of histone deacetylase inhibitors and cyclin-dependent kinase inhibitor flavopiridol
However, the in vivo efficacy of proteasome inhibitors on CML remains obscure, and whether proteasome inhibitors could exert synergistic/additive effects with IM needs more in-depth analysis. In this study, we investigated the  combined effects of BOR/PSI with IM on CML in vivo and in vitro. Intriguingly, the results showed that the combinatory regimens yielded enhanced therapeutic efficacies in CML murine models, potentiated effects on CML cells, and triggered Palbociclib positive feedback signal networks involving BCR-ABL, b-catenin, protein phosphatase 2A, NFkB and Bruton’s tyrosine kinase, suggesting potential benefits of IM/BOR for CML patients. IM at low concentration attenuates heart and kidney damages in hypertensive rats, prevents the development of atherosclerotic lesions and diabetes-induced inflammatory cytokine overexpression in the aorta, and reverse experimental pulmonary AMN107 Src-bcr-Abl inhibitor hypertension in mice. However, at high dose IM causes severe congestive heart failure in mice and in a small portion of patients. Furthermore, dynamics of CML disease progression suggests that additional agents will be beneficial to eradicate CML leukemia stem cells. Since cells expressing BCR-ABL showed significantly higher proteasome levels than did BCR-ABL-negative cells and were more sensitive to induction of apoptosis by proteasome inhibitor, we test the combined effects of IM and proteasome inhibitors and report here that in vivo IM/BOR combination causes an intensified therapeutic efficacy without obvious toxicity, providing an alternative option for CML treatment. We show that IM in combination with proteasome inhibitor significantly prolongs life span of BALB/c mice bearing BCRABL/GFP-expressing murine hematopoietic cells, and suppresses tumor growth in nude mice harboring K562 cells. In vitro, IM/BOR and IM/PSI exhibit an enhanced inhibition of long-term colony forming activity and short-term cell growth of CD34+ cells from CML patients at CP or BC, cause potentiated proliferation inhibition in K562 and 32D cells expressing BCR-ABL, and exert significantly potentiated apoptotic effects on CML cells. Heaney et al recently demonstrated that proteasome may be a relevant target for quiescent CML stem cells following tyrosine kinase inhibitor therapy, while proteasome inhibitor are capable of inducing CML stem cell specific apoptosis. Compared to normal cells, cancer cells often bear higher Dym and evade mitochondrial apoptosis. Normally, in response to cellular stress, the cell’s mitochondria are triggered to release cyto C into the cytosol which then binds to Apaf-1 and initiates the formation of apoptosome, leading to the activation of casp-9 and subsequent casp-3. The release of cyto C is tightly regulated by pro- and anti-apoptotic members of Bcl-2 family. In CML, BCR-ABL upregulates Bcl-2 and Bcl-XL through activation of STAT5, and inhibits release of cytochrome C and prevents caspase activation even after cyto C release, hence confering resistance to apoptosis to CML cells. Interestingly, IM/BOR and IM/PSI cause collapse of Dym, downregulation of pBCL-2, increase of cytoplasmic cyto C and activation of casp-9, -8 and -3. It is well-known that IM acts as a specific inhibitor of BCR-ABL. BOR and PSI significantly enhance IM-triggered suppression of pBCR-ABL and inhibition of its tyrosine kinase activity in vitro and in vivo. In consistence with a previous report, we show that activation of caspases by IM/ BOR and IM/PSI leads to catabolism of BCR-ABL, where caspase inhibitor not only reduces apoptosis but also inhibits degradation of BCR-ABL. IM/BOR and IM/PSI also downregulate pSTAT5. These data suggest that the combinatory regimens on one hand target the mitochondria, downregulate Bcl-2 and activate caspases, on the other hand inhibit BCR-ABL/STAT5 which might in turn potentiate downregulation of Bcl-2 and activation of caspases. Furthermore, activated caspases can enhance BCR-ABL catabolism and inactivation. Therefore, IM/BOR and IM/PSI may trigger a positive feedback apoptotic signaling network.
combined effects of BOR/PSI with IM on CML in vivo and in vitro. Intriguingly, the results showed that the combinatory regimens yielded enhanced therapeutic efficacies in CML murine models, potentiated effects on CML cells, and triggered Palbociclib positive feedback signal networks involving BCR-ABL, b-catenin, protein phosphatase 2A, NFkB and Bruton’s tyrosine kinase, suggesting potential benefits of IM/BOR for CML patients. IM at low concentration attenuates heart and kidney damages in hypertensive rats, prevents the development of atherosclerotic lesions and diabetes-induced inflammatory cytokine overexpression in the aorta, and reverse experimental pulmonary AMN107 Src-bcr-Abl inhibitor hypertension in mice. However, at high dose IM causes severe congestive heart failure in mice and in a small portion of patients. Furthermore, dynamics of CML disease progression suggests that additional agents will be beneficial to eradicate CML leukemia stem cells. Since cells expressing BCR-ABL showed significantly higher proteasome levels than did BCR-ABL-negative cells and were more sensitive to induction of apoptosis by proteasome inhibitor, we test the combined effects of IM and proteasome inhibitors and report here that in vivo IM/BOR combination causes an intensified therapeutic efficacy without obvious toxicity, providing an alternative option for CML treatment. We show that IM in combination with proteasome inhibitor significantly prolongs life span of BALB/c mice bearing BCRABL/GFP-expressing murine hematopoietic cells, and suppresses tumor growth in nude mice harboring K562 cells. In vitro, IM/BOR and IM/PSI exhibit an enhanced inhibition of long-term colony forming activity and short-term cell growth of CD34+ cells from CML patients at CP or BC, cause potentiated proliferation inhibition in K562 and 32D cells expressing BCR-ABL, and exert significantly potentiated apoptotic effects on CML cells. Heaney et al recently demonstrated that proteasome may be a relevant target for quiescent CML stem cells following tyrosine kinase inhibitor therapy, while proteasome inhibitor are capable of inducing CML stem cell specific apoptosis. Compared to normal cells, cancer cells often bear higher Dym and evade mitochondrial apoptosis. Normally, in response to cellular stress, the cell’s mitochondria are triggered to release cyto C into the cytosol which then binds to Apaf-1 and initiates the formation of apoptosome, leading to the activation of casp-9 and subsequent casp-3. The release of cyto C is tightly regulated by pro- and anti-apoptotic members of Bcl-2 family. In CML, BCR-ABL upregulates Bcl-2 and Bcl-XL through activation of STAT5, and inhibits release of cytochrome C and prevents caspase activation even after cyto C release, hence confering resistance to apoptosis to CML cells. Interestingly, IM/BOR and IM/PSI cause collapse of Dym, downregulation of pBCL-2, increase of cytoplasmic cyto C and activation of casp-9, -8 and -3. It is well-known that IM acts as a specific inhibitor of BCR-ABL. BOR and PSI significantly enhance IM-triggered suppression of pBCR-ABL and inhibition of its tyrosine kinase activity in vitro and in vivo. In consistence with a previous report, we show that activation of caspases by IM/ BOR and IM/PSI leads to catabolism of BCR-ABL, where caspase inhibitor not only reduces apoptosis but also inhibits degradation of BCR-ABL. IM/BOR and IM/PSI also downregulate pSTAT5. These data suggest that the combinatory regimens on one hand target the mitochondria, downregulate Bcl-2 and activate caspases, on the other hand inhibit BCR-ABL/STAT5 which might in turn potentiate downregulation of Bcl-2 and activation of caspases. Furthermore, activated caspases can enhance BCR-ABL catabolism and inactivation. Therefore, IM/BOR and IM/PSI may trigger a positive feedback apoptotic signaling network.