Tumor lymphangiogenesis was increased in VEGF-C MCF7 tumors injected in nude mice and lymph node metastasis were found more frequently. Similar results were reported with VEGF-C Evofosfamide overexpressing MDA-MB-435. In the present study, MCF7 cells overexpressing or not VEGF-C were inoculated into RAG-12/2 immunodeficient mice crossed with PAI-1 WT or PAI-1 deficient mice. In accordance with previous reports, we confirmed the increased growth rate of VEGF-C expressing tumors. The pro-tumoral effect of VEGF-C was previously attributed to a better oxygenation due to a slight angiogenic response or a decreased intratumoral pressure because of the increased number of lymphatic vessels. It is worth noting that the mice background and the immunodeficiency rate are essential factors influencing the lymph node dissemination. Indeed, the propensity of VEGF-C expressing cells to disseminate into lymph node was higher in nude micethan in SCID miceor RAG-12/2 mice. Since these mice differ in their B-lymphocyte status, it suggests that B lymphocytes might contribute to lymph node dissemination of cancerous cells. Accordingly, the requirement of B-lymphocytes was also observed in a lymphangiogenesis model of mycoplasma infection of the pulmonary tract. In agreement with previous studies, PAI-1 deficiency was associated with decreased tumor development. We further analysed the lymphatic invasion of these tumors and their dissemination into lymph nodes. Although VEGF-C expression led to an enhancement of lymphatic vessel numbers, no difference was observed in PAI-1 WT and PAI-12/2 mice. Moreover, both genotypes showed a similar rate of lymph node metastasis. These data clearly demonstrate that PAI-1 is 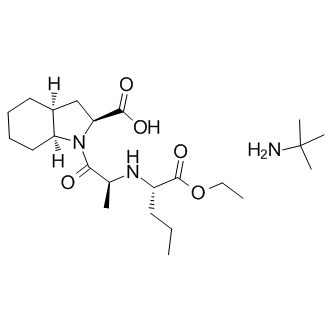 not implicated in tumoral lymphangiogenesis. Moreover, our data are in line with a previous study on PyMT transgenic mice showing that the primary tumor growth was not significantly affected by PAI-1 deficiency and neither was the lung metastatic burden. We now demonstrate that PAI-1 is dispensable for tumoral lymphangiogenesis by using the PyMT and PAI-1 double transgenic mice. Knowing that inflammation influences cancer progressionand that lymphangiogenesis and inflammation processes are closely related, we applied a model of lymphangioma to PAI12/2 mice. This system consists in a benign hyperplasia of lymphatic vessels induced by the injection of Freund adjuvant and is often used to isolate lymphatic endothelial cells. In this system, the inflammatory reaction induced by Freund adjuvant relies on the recruitment of leukocytes by cytokines secreted by cells of the peritoneum. In PAI-1 deficient mice, we observed a macroscopic decrease of the lymphangioma formation as compared to PAI-1 WT mice. This effect could be ascribed to a reduction of fibrosis rather than to a decrease in lymphatic vessel recruitment. Accordingly, PAI-1 deficiency slowed down the fibrotic reaction in different modelsby accelerating plasmin-mediated proteolysisor by influencing macrophage or AP24534 myofibroblast recruitment. The lack of PAI-1 effect on inflammation related lymphangiogenesis was further confirmed by similar injury-induced corneal lymphangiogenesis observed in PAI-12/2 and PAI-1 WT mice. The increased lymphatic vessel size observed in lymphangioma of PAI-12/2 mice is intriguing. However, note that this variation in vessel structure is associated with a reduction of matrix deposition which may influence vessel branching. Studies on mammary gland morphogenesis revealed that the collagen deposition inhibition reduced developing tubular structure bifurcations. The matrix proteolytic breakdown could compromise the scaffold mechanical integrity necessary to counter endothelial cells-generated forces during the tube formation process.
not implicated in tumoral lymphangiogenesis. Moreover, our data are in line with a previous study on PyMT transgenic mice showing that the primary tumor growth was not significantly affected by PAI-1 deficiency and neither was the lung metastatic burden. We now demonstrate that PAI-1 is dispensable for tumoral lymphangiogenesis by using the PyMT and PAI-1 double transgenic mice. Knowing that inflammation influences cancer progressionand that lymphangiogenesis and inflammation processes are closely related, we applied a model of lymphangioma to PAI12/2 mice. This system consists in a benign hyperplasia of lymphatic vessels induced by the injection of Freund adjuvant and is often used to isolate lymphatic endothelial cells. In this system, the inflammatory reaction induced by Freund adjuvant relies on the recruitment of leukocytes by cytokines secreted by cells of the peritoneum. In PAI-1 deficient mice, we observed a macroscopic decrease of the lymphangioma formation as compared to PAI-1 WT mice. This effect could be ascribed to a reduction of fibrosis rather than to a decrease in lymphatic vessel recruitment. Accordingly, PAI-1 deficiency slowed down the fibrotic reaction in different modelsby accelerating plasmin-mediated proteolysisor by influencing macrophage or AP24534 myofibroblast recruitment. The lack of PAI-1 effect on inflammation related lymphangiogenesis was further confirmed by similar injury-induced corneal lymphangiogenesis observed in PAI-12/2 and PAI-1 WT mice. The increased lymphatic vessel size observed in lymphangioma of PAI-12/2 mice is intriguing. However, note that this variation in vessel structure is associated with a reduction of matrix deposition which may influence vessel branching. Studies on mammary gland morphogenesis revealed that the collagen deposition inhibition reduced developing tubular structure bifurcations. The matrix proteolytic breakdown could compromise the scaffold mechanical integrity necessary to counter endothelial cells-generated forces during the tube formation process.
Monthly Archives: July 2019
Involved in HER2 crosstalk may lead to the development of new strategies for the treatment of HER2
Fatty acid synthaseis the enzyme that is responsible for the cellular synthesis of palmitate. As a metabolic oncogene, FASN is constitutively overexpressed and hyperactive in aggressive breast carcinoma. The up-regulation of FASN in tumors is an early and nearly universal epigenetic change that is involved in the development, maintenance and enhancement of the malignant phenotype. We hypothesized that FASN was the key downstream MK-1775 effector of the bidirectional ER/HER2 crosstalk that promotes malignant phenotypes, such as proliferation, migration, apoptosis evasion and endocrine resistance, in ER+/HER2+ breast cancer cells. There is bidirectional crosstalk between FASN and HER2 in cancer cells.FASN overexpression positively correlates with HER2 amplificationin breast cancer cells. FASN is the downstream mediator of HER2 tumorigenicity and cancer progression. FASN inhibition decreases HER2 expression by upregulating PEA3, a HER2 transcriptional inhibitor, and by changing the lipid composition and function of tumor cell membranes, thereby altering the cellular localization of HER2. In addition, inhibiting FASN negatively affects the interaction between EGFR and HER2, which is a mechanism of trastuzumab resistance in breast cancer. FASN is regulated by estrogen in ER-positive breast cancer cells; estrogen 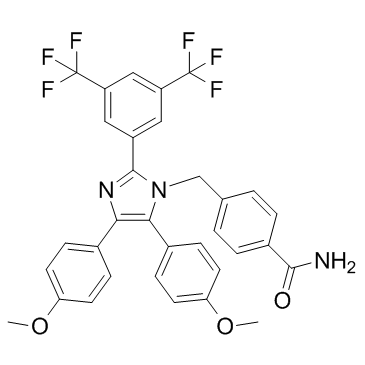 stimulates FASN expression. FASN expression is part of the E2-mediatedcellular response that leads to the proliferation of hormone-dependent carcinoma cells. Inhibiting FASN dramatically augments E2-stimulated, ER-driven transcriptional activity, synergistically enhances the E2-mediated down-regulation of ER expression and impairs E2-induced nuclear accumulation of ER. Furthermore, inhibiting FASN induces antitumor activity by acting as a SERM in ER-positive breast cancer cells.Therefore, FASN is most likely the downstream effector underlying ER/HER2 crosstalk in dual-positive breast cancer, but the signaling pathway that is involved remains unknown. The mammalian target of rapamycinsignaling pathway is one of the most important pathways in signal transduction in cancer. mTOR is a serine/threonine-specific kinase that is responsible for mitogen-induced cell proliferation, survival and motility in cancer cells. The mTOR signaling pathway may connect ER/HER2 crosstalk with the downstream effector FASN. HER2amplification activates the mTOR signaling pathway. Inhibiting mTOR blocks multiple stages of HER2induced tumorigenic progression and improves the antitumor activity of HER2 inhibitors.The mTOR pathway is also related to endocrine therapy resistance. Cycloheximide SERM-resistant MCF-7/ HER2 cells up-regulate mTOR expression by activating the PI3K/AKT, ERK and MAPK signaling pathways. The activated Phosphoinositide 3-kinase /AKT pathway stimulates mTOR to phosphorylate its downstream effectors p70 ribosomal S6 kinaseand eukaryotic initiation factor 4E binding protein 1, mediating the expression of genes associated with tumor malignancy. Therefore, the combination of mTOR inhibitors and hormone- or HER2-targeting therapies was believed to be a promising strategy for overcoming initial therapeutic resistance and for preventing the development of resistance in ER+/HER2+ breast cancer.There is an intimate relationship between FASN and mTOR. mTOR activation induces FASN expression, and inhibiting the mTOR signaling pathway down-regulates FASN expression. FASN inhibition upregulates DDIT4, a negative regulator of the mTOR pathway, suggesting that FASN inhibition negatively regulates the mTOR pathway via DDIT4. In this study, we found that FASN was overexpressed in ER+/ HER2+ breast cancer cells. And the transcriptional activity of FASN promoter was high in ER+/HER2+ breast cancer cells.
stimulates FASN expression. FASN expression is part of the E2-mediatedcellular response that leads to the proliferation of hormone-dependent carcinoma cells. Inhibiting FASN dramatically augments E2-stimulated, ER-driven transcriptional activity, synergistically enhances the E2-mediated down-regulation of ER expression and impairs E2-induced nuclear accumulation of ER. Furthermore, inhibiting FASN induces antitumor activity by acting as a SERM in ER-positive breast cancer cells.Therefore, FASN is most likely the downstream effector underlying ER/HER2 crosstalk in dual-positive breast cancer, but the signaling pathway that is involved remains unknown. The mammalian target of rapamycinsignaling pathway is one of the most important pathways in signal transduction in cancer. mTOR is a serine/threonine-specific kinase that is responsible for mitogen-induced cell proliferation, survival and motility in cancer cells. The mTOR signaling pathway may connect ER/HER2 crosstalk with the downstream effector FASN. HER2amplification activates the mTOR signaling pathway. Inhibiting mTOR blocks multiple stages of HER2induced tumorigenic progression and improves the antitumor activity of HER2 inhibitors.The mTOR pathway is also related to endocrine therapy resistance. Cycloheximide SERM-resistant MCF-7/ HER2 cells up-regulate mTOR expression by activating the PI3K/AKT, ERK and MAPK signaling pathways. The activated Phosphoinositide 3-kinase /AKT pathway stimulates mTOR to phosphorylate its downstream effectors p70 ribosomal S6 kinaseand eukaryotic initiation factor 4E binding protein 1, mediating the expression of genes associated with tumor malignancy. Therefore, the combination of mTOR inhibitors and hormone- or HER2-targeting therapies was believed to be a promising strategy for overcoming initial therapeutic resistance and for preventing the development of resistance in ER+/HER2+ breast cancer.There is an intimate relationship between FASN and mTOR. mTOR activation induces FASN expression, and inhibiting the mTOR signaling pathway down-regulates FASN expression. FASN inhibition upregulates DDIT4, a negative regulator of the mTOR pathway, suggesting that FASN inhibition negatively regulates the mTOR pathway via DDIT4. In this study, we found that FASN was overexpressed in ER+/ HER2+ breast cancer cells. And the transcriptional activity of FASN promoter was high in ER+/HER2+ breast cancer cells.
However FLT3 kinase targeting by mono-therapy with curren apoptosis in Src inhibitor-resistant prostate cancer cells
This is likely through the inhibition of Etk and down regulation of Myc and BCL2. The more detailed signaling pathways between the link remains to be further investigated. In summary, we have identified an Etk and Src dual inhibitor, CTA095, with good selectivity toward prostate cancer cells. This inhibitor could overcome Src inhibitor resistance and induce apoptosis in Src inhibitor-resistant prostate cancer cells. This study indicates that Etk and Src dual inhibition holds exceptional promise as a novel treatment strategy for prostate cancer. Acute myeloid leukemiais the most common hematologic malignancy in adults with a high incidence rate and low survival probability. AML progresses rapidly due to the rapid growth of abnormal white blood cells that accumulate in the bone marrow and interfere with the production of red blood cells, platelets, and normal white blood cells. If left untreated, AML is usually fatal within weeks or months after diagnosis. FLT3, a cell surface receptor belonging to the class III receptor 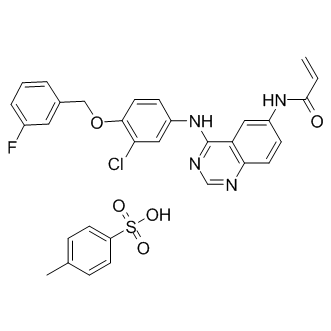 tyrosine kinase family, plays a pivotal role in the differentiation and survival of the hematopoietic stem cells in bone marrow. FLT3 is one of the most commonly mutated genes in AML. Activating FLT3 mutations, FLT3-ITDand FLT3-TKD, are frequently observed in approximately 30% of adult AML patients. FLT3-activating mutantions critically regulate leukemic transformation by accelerating proliferation and suppressing apoptosis and are significantly associated with poor prognosis. These findings highlight FLT3-ITD and FLT3-TKD as highly attractive therapeutic targets for drug development in human AML. There are now several NVP-BKM120 distributor classes of small molecule FLT3 inhibitors that have entered clinical trials. However, effective drugs have not yet been identified in clinics. Although these inhibitors have demonstrated promising anti-cancer activity in in vitro and in vivo preclinical models, clinically positive responses in AML patients receiving single-agent FLT3 inhibitors are limited due to the transient reduction of peripheral blasts but not bone marrow blasts or the occurrence of inhibitor-resistant FLT3 mutations in patients. Therefore, combinatorial strategies of FLT3 inhibitors and other chemotherapeutic agents may be beneficial approaches to improve FLT3 inhibitor therapy and to overcome treatment failures. The FLT3 inhibitor CEP701combined with standard AML chemotherapeutic agents has the potential to improve clinical outcomes in AML patients. In addition, histone deacetylase inhibitors, a class of compounds that can induce cancer cell growth arrest and cell death by altering the acetylation status of both histone and non-histone proteins, can enhance the activity of FLT3 inhibitors on AML cell apoptosis. The HDACi vorinostatexhibits clinical activity in AML; however, its efficacy as a single agent is only WY 14643 moderate. In this study, we report data characterizing the pharmacological profile of a new FLT3 kinase inhibitor, BPR1J-340, and elucidate the possible molecular mechanism of the strongly synergistic effects in combination with SAHA in FLT3-ITD + cells. These results highlight the therapeutic potential of BPR1J-340 and SAHA in AML and support its preclinical or clinical development. Given that the abnormal expression of FLT3 kinase, including amplified or aberrantly activated FLT3, is frequently observed in the blast cells of AML patients, FLT3 represents an attractive therapeutic target of choice for drugs development in AML. To date, several potential FLT3 inhibitors have been developed and examined in AML patients, including lestaurtiniband midostaurinin phase III clinical trials and sunitinib malate, sorafenib, quizartinib, and crenolanibin phase II trials.
tyrosine kinase family, plays a pivotal role in the differentiation and survival of the hematopoietic stem cells in bone marrow. FLT3 is one of the most commonly mutated genes in AML. Activating FLT3 mutations, FLT3-ITDand FLT3-TKD, are frequently observed in approximately 30% of adult AML patients. FLT3-activating mutantions critically regulate leukemic transformation by accelerating proliferation and suppressing apoptosis and are significantly associated with poor prognosis. These findings highlight FLT3-ITD and FLT3-TKD as highly attractive therapeutic targets for drug development in human AML. There are now several NVP-BKM120 distributor classes of small molecule FLT3 inhibitors that have entered clinical trials. However, effective drugs have not yet been identified in clinics. Although these inhibitors have demonstrated promising anti-cancer activity in in vitro and in vivo preclinical models, clinically positive responses in AML patients receiving single-agent FLT3 inhibitors are limited due to the transient reduction of peripheral blasts but not bone marrow blasts or the occurrence of inhibitor-resistant FLT3 mutations in patients. Therefore, combinatorial strategies of FLT3 inhibitors and other chemotherapeutic agents may be beneficial approaches to improve FLT3 inhibitor therapy and to overcome treatment failures. The FLT3 inhibitor CEP701combined with standard AML chemotherapeutic agents has the potential to improve clinical outcomes in AML patients. In addition, histone deacetylase inhibitors, a class of compounds that can induce cancer cell growth arrest and cell death by altering the acetylation status of both histone and non-histone proteins, can enhance the activity of FLT3 inhibitors on AML cell apoptosis. The HDACi vorinostatexhibits clinical activity in AML; however, its efficacy as a single agent is only WY 14643 moderate. In this study, we report data characterizing the pharmacological profile of a new FLT3 kinase inhibitor, BPR1J-340, and elucidate the possible molecular mechanism of the strongly synergistic effects in combination with SAHA in FLT3-ITD + cells. These results highlight the therapeutic potential of BPR1J-340 and SAHA in AML and support its preclinical or clinical development. Given that the abnormal expression of FLT3 kinase, including amplified or aberrantly activated FLT3, is frequently observed in the blast cells of AML patients, FLT3 represents an attractive therapeutic target of choice for drugs development in AML. To date, several potential FLT3 inhibitors have been developed and examined in AML patients, including lestaurtiniband midostaurinin phase III clinical trials and sunitinib malate, sorafenib, quizartinib, and crenolanibin phase II trials.
To avoid ineffective high marine actinobacteria for activity under in vitro and in vivo conditions
The first auditory neurons, the spiral ganglion neurons, connect the hair cells of the auditory system with higher regions of the central auditory pathway. Interactions between inner hair cells and afferent fibres of the SGN occur in terms of signal transmission via glutamate release from depolarized hair cells and in terms of trophic support with growth factors like BDNF and NT3 delivered from the hair cells. Both kinds of interaction are essential for the maintenance of the homeostasis and functionality of the SGN. Therefore, age-, drug- and noise-induced damage and loss of the hair cells consequently causes a successive secondary degeneration of the SGN due to the absence of functional innervation and deprived neurotrophic support. However, recent evidence shows that SGN degeneration in humans is not dependent on hair cell loss. In addition, using a mouse model, the role of supporting cells in the maintenance of SGN was demonstrated. One therapeutic measure to moderate or compensate the loss of the hair cells is the treatment with a cochlear implant that directly stimulates residual SGN. Although this is an ongoing controversial discussion, it is still believed that the benefit of such a cochlear implant strongly depends not only on the excitability of the SGN, but also on the number of surviving neurons. Thus, current research focuses on the preservation of unaffected and the regeneration of deprived SGN in addition to the electrical innervation provided by the cochlear implant. A potent approach to increase the viability of SGN in vitro and in vivo is the external application of BDNF. In the cochlea, 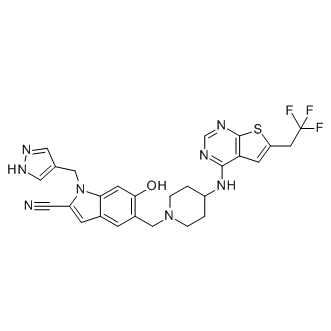 the protective effect of BDNF is primarily promoted by the activation of the high-affinity tyrosine kinase receptor B. TrkB signals via an intracellular cascade that is connected to the extracellular signal-regulated kinase /mitogen-activated protein kinase pathway. This finally induces the phosphorylation and thereby activation of the cyclic adenosine monophosphate -response element-binding protein. CREB in turn triggers the expression of survival promoting genes within the SGN. Another important activator for CREB-mediated neuroprotection is cAMP. The multifunctional second messenger cAMP promotes neuronal differentiation and survival as well as outgrowth, regeneration and guidance of neuronal processes. Carefully increased concentrations of cAMP, as evoked by the application of cAMP analogues, promote the survival and enhance fibre elongation of SGN in vitro. Another more clinically relevant option to increase cAMP levels in neurons is the application of specific phosphodiesterase type 4 inhibitors such as Rolipram. So far, several studies have demonstrated neuroprotective and anti-inflammatory effects of LY2157299 Rolipram after lesions of the central nervous system. Additionally, neuroregeneration and axonal outgrowth can be enhanced by Rolipram application. Different studies reported that its beneficial effects can be enhanced when applied in combination with other protective factors or substances. In order to exert its neuroprotective effect, Rolipram increases the level of intracellular cAMP. As recently demonstrated by Xu et al., 2012, the beneficial effect of intracellular cAMP on SGN critically depends on low cAMP concentrations. A previous study of our group demonstrated a protective effect on SGN in vitro only if Rolipram was delivered encapsulated in lipid nanocapsules. However, this neuroprotective effect was not FTY720 observed after treatment with pure Rolipram. One explanation could be that the used Rolipram concentration induced an increase of cAMP too high to promote the protective effect demonstrated by cAMP analogues. Therefore, the aim of the present study was to clarify if a Rolipram-induced increase of cAMP can be clinically relevant for the protection of SGN.
the protective effect of BDNF is primarily promoted by the activation of the high-affinity tyrosine kinase receptor B. TrkB signals via an intracellular cascade that is connected to the extracellular signal-regulated kinase /mitogen-activated protein kinase pathway. This finally induces the phosphorylation and thereby activation of the cyclic adenosine monophosphate -response element-binding protein. CREB in turn triggers the expression of survival promoting genes within the SGN. Another important activator for CREB-mediated neuroprotection is cAMP. The multifunctional second messenger cAMP promotes neuronal differentiation and survival as well as outgrowth, regeneration and guidance of neuronal processes. Carefully increased concentrations of cAMP, as evoked by the application of cAMP analogues, promote the survival and enhance fibre elongation of SGN in vitro. Another more clinically relevant option to increase cAMP levels in neurons is the application of specific phosphodiesterase type 4 inhibitors such as Rolipram. So far, several studies have demonstrated neuroprotective and anti-inflammatory effects of LY2157299 Rolipram after lesions of the central nervous system. Additionally, neuroregeneration and axonal outgrowth can be enhanced by Rolipram application. Different studies reported that its beneficial effects can be enhanced when applied in combination with other protective factors or substances. In order to exert its neuroprotective effect, Rolipram increases the level of intracellular cAMP. As recently demonstrated by Xu et al., 2012, the beneficial effect of intracellular cAMP on SGN critically depends on low cAMP concentrations. A previous study of our group demonstrated a protective effect on SGN in vitro only if Rolipram was delivered encapsulated in lipid nanocapsules. However, this neuroprotective effect was not FTY720 observed after treatment with pure Rolipram. One explanation could be that the used Rolipram concentration induced an increase of cAMP too high to promote the protective effect demonstrated by cAMP analogues. Therefore, the aim of the present study was to clarify if a Rolipram-induced increase of cAMP can be clinically relevant for the protection of SGN.
Revealed that BTZ resistant SKM1R cells are characterized by upregulation of ERK
These findings indicate that upregulation of ERK activity may be a potential mechanism for BTZ resistance. MEK/ERK pathway is activated in aggressive CKS1B-overexpressing plasma cell myeloma cells. Higher expression levels of p-ERK in human prostate cancer samples were associated with tumor progression. Hyperactivation of the MAPK pathway has also been implicated in the development of neurofibromatosis type Vemurafenib 1-associated leukemia. Our findings are consistent with these previous reports. We further showed that resistance to BTZ can be reversed by PD98059 and U0126, suggesting that MEK/ERK pathway may be a potential target for MDS patients who developed resistance to BTZ. Our findings are in line with previous report that ERK inhibitors synergized with BTZ on anticancer effects in medulloblastoma cells. Using the MUTZ-1 cell line, Huang et al found no notable changes in p-ERK1/2 after BTZ treatment. The difference in p-ERK expression in response to BTZ treatment may be explained by the nature of the two different cell lines. MUTZ-1 was isolated from the malignant cells of a 5-year-old girl with MDS, whereas SKM-1 was established from leukemic cells of a 76year-old patient. These two cell lines exhibit different chromosome abnormalities. Autophagy is a mechanism that degrades dysfunctional cellular components through lysosomes. The activation of autophagy may lead to either cell death or increased survival depending on cell types and conditions. For example, BTZ-induced autophagy led to cell death in MDS/AML cells. Consistent with these previous findings, our present study showed that BTZ induced the conversion of LC3I to LC3II in wild type SKM-1 cells but not in -Pugnac-chemical-structure.gif) BTZ resistance SKM-1R cells. Our results suggest that autophagic cell death may contribute, at least in part, to BTZinduced cell cycle arrest and apoptosis. In some cases, the LC3-I to LC3-II conversion is necessary but not sufficient to trigger cell autophagy. Further studies are needed to ascertain the role of autophagy in BTZ induced cell death by silencing autophagy related proteins such as ATG5, ATG7 and ATG8 in Skm-1 cells. In conclusion, the present study demonstrates that BTZ induces cell cycle arrest, apoptosis and autophagy in SKM-1 cells. The cytotoxic effects of BTZ appeared to depend, at least in part, on the inhibition of the MEK/ERK pathway. The BTZ resistant SKM-1R cells are characterized by upregulation of ERK. Based on the present observations, a combinational therapy of BTZ and MAPK inhibitors may be an effective complement to current therapeutic approaches for MDS. Cyclin-dependent kinase inhibitors negatively regulate the cell cycle, helping to set the threshold for cell cycle entry and promoting exit from cell cycle in response to growth factor withdrawal, inhibitory cytokines, contact inhibition, DNA damage, and senescence. Over-expression of these factors can impose cell cycle arrest in a retinoblastoma protein -dependent manner, while elimination of CDK inhibitors leads to enhanced proliferative responses in many types of tissues and cell types. CDK inhibitors are subdivided into two groups based on functional and structural differences. Three different approaches were considered for the BI-D1870 control of more virulent malarial parasite, Plasmodium falciparum. They are widely exploited development of effective vaccines, vector control and development of new drugs. It is very difficult to develop a vaccine due to their exhibition of their multiple antigenicity. Based on several factors, the vector control shows limited success. On the other hand, there is an increasing resistance of malarial parasites to the existing drug hence, there is an urgent need demand for new antimalarial agents.
BTZ resistance SKM-1R cells. Our results suggest that autophagic cell death may contribute, at least in part, to BTZinduced cell cycle arrest and apoptosis. In some cases, the LC3-I to LC3-II conversion is necessary but not sufficient to trigger cell autophagy. Further studies are needed to ascertain the role of autophagy in BTZ induced cell death by silencing autophagy related proteins such as ATG5, ATG7 and ATG8 in Skm-1 cells. In conclusion, the present study demonstrates that BTZ induces cell cycle arrest, apoptosis and autophagy in SKM-1 cells. The cytotoxic effects of BTZ appeared to depend, at least in part, on the inhibition of the MEK/ERK pathway. The BTZ resistant SKM-1R cells are characterized by upregulation of ERK. Based on the present observations, a combinational therapy of BTZ and MAPK inhibitors may be an effective complement to current therapeutic approaches for MDS. Cyclin-dependent kinase inhibitors negatively regulate the cell cycle, helping to set the threshold for cell cycle entry and promoting exit from cell cycle in response to growth factor withdrawal, inhibitory cytokines, contact inhibition, DNA damage, and senescence. Over-expression of these factors can impose cell cycle arrest in a retinoblastoma protein -dependent manner, while elimination of CDK inhibitors leads to enhanced proliferative responses in many types of tissues and cell types. CDK inhibitors are subdivided into two groups based on functional and structural differences. Three different approaches were considered for the BI-D1870 control of more virulent malarial parasite, Plasmodium falciparum. They are widely exploited development of effective vaccines, vector control and development of new drugs. It is very difficult to develop a vaccine due to their exhibition of their multiple antigenicity. Based on several factors, the vector control shows limited success. On the other hand, there is an increasing resistance of malarial parasites to the existing drug hence, there is an urgent need demand for new antimalarial agents.