Surveillance bias might also have occurred because patients with POAG might be more likely to visit clinics than patients without POAG are. However, we used propensity scores to balance the incidences of major underlying diseases between the POAG and control patients. Therefore, the need for medical attention was likely similar between the POAG and control patients, resulting in a similar likelihood of having AD detected. The impact of surveillance bias on the risk of AD in POAG patients was likely minimal. However, the surveillance bias could still be a factor affected the risk of PD and AD. In conclusion, our population-based study demonstrated that POAG is a significant predictor for the development of AD, but POAG is not a predictor of PD. Future studies are warranted to confirm our results, and identify the underlying pathological mechanism that contributes to the development of AD in POAG patients. Tubeimoside-I astrocytes and oligodendrocytes are macroglial cells found in all regions of the central nervous system. It is estimated that macroglial cells constitute as many as 90% of cells in some regions of the CNS, with astrocytes being the predominant cell type. Although both glial cell types act to support the activities of neurons, oligodendrocytes and astrocytes have clearly distinct functions. The primary function of an oligodendrocyte is to form myelin sheaths around multiple axons for rapid transmission of electrical pulses along axons. In contrast, astrocytes play many diverse roles, both supportive and active, in the functioning of the CNS. Among those are the regulation of ion and neurotransmitter concentrations, formation of the brain blood barrier, modulation of synapse formation and efficacy, and induction of neurogenesis in the adult brain. Thus, it is unsurprising that abnormalities in astrocyte density and functioning have recently been implicated in several common neurological diseases including neuropathic pain, depression, and schizophrenia. Spinal cord has served as an excellent model (R)-(-)-Modafinic acid system to study the origin and molecular specification of neurogenesis and gliogenesis due to its relatively simple anatomy and structures. In the developing spinal cord, neuroepithelial cells in the ventricular zone first give rise to neurons which subsequently migrate away from the ventricular zone by radial migration. At later stages, neuroepithelial cells switch to produce glial cells, i.e. astrocytes or oligodendrocytes. In the past decade, great progress has been made in our understanding of the origin and molecular control of oligodendrocyte development. During gliogenesis, early oligodendrocyte progenitor cells originate from the ventral motor neuron progenitor domain of the ventral neuroepithelium, but a small number of OPCs are also generated from dorsal neural progenitor cells at later stage. Recent molecular and genetic evidence suggests that astrocytes are produced from other domains of neural progenitor cells, particularly from the p1-p3 domains in the ventral spinal cord. Although considerable progress has been made in our understanding of the molecular specification of oligodendrocytes, the molecular mechanisms that control the development of astrocytes have been lagging, partly due to the lack of well-defined stage-specific markers for astrocyte lineage. The Nkx6.1 homeobox 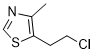 gene is expressed in the ventral neural progenitor cells that give rise to motor, V2, and V3 neurons and ventral oligodendrocytes.
gene is expressed in the ventral neural progenitor cells that give rise to motor, V2, and V3 neurons and ventral oligodendrocytes.
Mutation exhibit a switch in the identity of progenitor cells with a ventral competitive model resulted
Leave a reply