On post-translational activation and stability of cell cycle regulatory proteins. To identify downstream molecular actors involved in PTEN mediated cell cycle modulation, a prostatic cancer cellular model expressing PTEN cDNA was recently used. This modification led to a significant inhibition of cell proliferation due to cycle arrest in the G1 phase. Gene expression analysis subsequent to PTEN reintroduction, coupled with phosphorylation status of downstream molecular targets, suggest that PTEN physiologically regulates cell cycle related proteins, through pAKT dependent and independent ways. E2F2, cdc25a, Cyclin G2 and RBL2 proteins are among them. The aim of the present study was to shed light on the role of PTEN pathway on cell cycle regulation in GSCs through a cellular differentiation model. Phosphoproteomics profiling of different GSCs lines, using reverse phase protein microarrays, indicates a heterogeneous basal activity of PTEN and related molecules. Transcriptomics profiling of GSCs induced to differentiate demonstrates specific molecular changes in PTEN positive cells, that are not observed in cells lacking its activation. The most modulated transcripts suggest a post-transcriptional regulation model, which involves cdc25a as the main target. A diagnosis of glioblastoma multiforme, established histologically according to the WHO classification, was the eligibility criteria. Patients AbMole Metyrapone characteristics are summarized in table 1. Tumor samples were obtained by complete or partial resection before the initiation of treatment with radiation and chemotherapy. The expression of both the proliferation marker Ki-67 and of tumor protein 53 were characterized on tumor specimen by 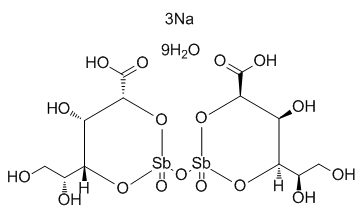 immunohistochemistry on deparaffinized sections using the avidin-biotin-peroxidase complex methods, anti-p53 monoclonal antibody and AbMole Mepiroxol anti-epidermal growth factor receptor monoclonal antibody. Tumors were considered p53 deficient if immunoreaction stained the nuclei of less than 5% of cells. Tumors showing moderate-to-strong immunostaining for EGFR in.20% of cells were considered EGFR positive. O6-methylguanine-DNA methyltransferase promoter methylation patterns were assessed on genomic DNA extracted from paraffin-embedded tissue by methylation-specific: it has been shown that the DNA repair protein MGMT influence the resistance of glioblastoma cells to alkylating agents, such as nitrosoureas and temozolomide thus representing and important prognostic and therapeutic indicator. Stem cells tightly regulate cell cycle progression in order to remain quiescent inside their cellular niche with progress occurring only when external stimuli indicates the need for cell growth or for the replacement of damaged or senescent cells.
immunohistochemistry on deparaffinized sections using the avidin-biotin-peroxidase complex methods, anti-p53 monoclonal antibody and AbMole Mepiroxol anti-epidermal growth factor receptor monoclonal antibody. Tumors were considered p53 deficient if immunoreaction stained the nuclei of less than 5% of cells. Tumors showing moderate-to-strong immunostaining for EGFR in.20% of cells were considered EGFR positive. O6-methylguanine-DNA methyltransferase promoter methylation patterns were assessed on genomic DNA extracted from paraffin-embedded tissue by methylation-specific: it has been shown that the DNA repair protein MGMT influence the resistance of glioblastoma cells to alkylating agents, such as nitrosoureas and temozolomide thus representing and important prognostic and therapeutic indicator. Stem cells tightly regulate cell cycle progression in order to remain quiescent inside their cellular niche with progress occurring only when external stimuli indicates the need for cell growth or for the replacement of damaged or senescent cells.
Monthly Archives: April 2019
Evidence suggests that actin assembly at the cell-cell interaction site is nucleated by the Arp2/3 complex
Their results showed that TCR-induced PI3K/Akt activation needs both membrane translocation and tyrosine phosphorylation of SLP-76 as well as LAT for the localization of SLP-76 to the membrane. A critical role for both LAT and SLP-76 has been demonstrated through reconstitution of LAT-deficient Jurkat T cell lines with LAT or SLP-76 deliberately targeted to the rafts. We have found lower AbMole Aristolochic-acid-A c-value for SLP-76 in an unperturbed TCR signaling AbMole Diperodon pathway. In contrast, it has became 0.60307 and 0.5495 in pathogen perturbed system and in the system, where it is optimized for two conflicting objective functions, respectively. In the same manner, we have found lower c-value for LAT in an unperturbed TCR signaling pathway. In contrast, it has become 0.57508 and 0.15188 in pathogen perturbed system and in the system, where it 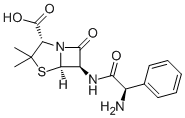 is optimized for two conflicting objective functions, respectively. In LAT-deficient J.Cam2 and ANJ3 cells, PLCc1, VAV and SLP76 phosphorylation were not detected and TCR-mediated signaling events were found to be impaired. Early T cell development has been found to be blocked in LAT-negative mice similar to that observed in mice, in which either LCK and FYN, or ZAP70 and Syk was deleted. These observations demonstrate that LAT is an obligatory step, downstream of LCK and ZAP70, and is an important link to Ras/ MAP kinase and PLCc1 pathways. Dlg1 is expressed in all T cell developmental stages and forms a stable complex with LCK. It functions as a negative regulator of T cell activation. Endogenous Dlg1 connects with TCRf and CBL. The transient overexpression of Dlg1 attenuates basal and VAV1-induced NFAT reporter activation. It is also shown that the reduction of Dlg1 expression by RNA interference enhances both CD3- and SAg-mediated NFAT activation. Thus it is interpreted that Dlg1 acts as an activation antagonist. This is the reason, we have got higher c-value for Dlgh1 in an unperturbed TCR signaling pathway. In contrast, it has become 0 and 0.46094 in pathogen perturbed system and in the system, where it is optimized for two conflicting objective functions, respectively. The cytoskeleton of eukaryotic cells has a pivotal role in hostpathogen interactions as it is essential for epithelial and endothelial barrier functions. It limits the invasion and dissemination of pathogens in tissues. GTP-binding proteins of the Rho family are regulators of the actin cytoskeleton, and Rho proteins play essential roles in host cell invasion by bacteria. They are the targets for bacterial protein toxins that either inactivate GTPases by ADP-ribosylation or glucosylation, or activate them by deamidation. Specifically, Rho GTPase Cdc42 regulates cytoskeletal changes at the immunological synapse that are critical to T cell activation.
is optimized for two conflicting objective functions, respectively. In LAT-deficient J.Cam2 and ANJ3 cells, PLCc1, VAV and SLP76 phosphorylation were not detected and TCR-mediated signaling events were found to be impaired. Early T cell development has been found to be blocked in LAT-negative mice similar to that observed in mice, in which either LCK and FYN, or ZAP70 and Syk was deleted. These observations demonstrate that LAT is an obligatory step, downstream of LCK and ZAP70, and is an important link to Ras/ MAP kinase and PLCc1 pathways. Dlg1 is expressed in all T cell developmental stages and forms a stable complex with LCK. It functions as a negative regulator of T cell activation. Endogenous Dlg1 connects with TCRf and CBL. The transient overexpression of Dlg1 attenuates basal and VAV1-induced NFAT reporter activation. It is also shown that the reduction of Dlg1 expression by RNA interference enhances both CD3- and SAg-mediated NFAT activation. Thus it is interpreted that Dlg1 acts as an activation antagonist. This is the reason, we have got higher c-value for Dlgh1 in an unperturbed TCR signaling pathway. In contrast, it has become 0 and 0.46094 in pathogen perturbed system and in the system, where it is optimized for two conflicting objective functions, respectively. The cytoskeleton of eukaryotic cells has a pivotal role in hostpathogen interactions as it is essential for epithelial and endothelial barrier functions. It limits the invasion and dissemination of pathogens in tissues. GTP-binding proteins of the Rho family are regulators of the actin cytoskeleton, and Rho proteins play essential roles in host cell invasion by bacteria. They are the targets for bacterial protein toxins that either inactivate GTPases by ADP-ribosylation or glucosylation, or activate them by deamidation. Specifically, Rho GTPase Cdc42 regulates cytoskeletal changes at the immunological synapse that are critical to T cell activation.
The role of SLP-76 and LAT in the activation of PI3K signaling conflicting objective functions
A study has shown a requirement for the LCKSH3 domain in optimal T cell development. Biochemical studies have indicated that LCK plays a key role in initiating TCR signals through receptor phosphorylation and activation of ZAP70 tyrosine kinase. CD45 tyrosine phosphatase maintains the inhibitory C-terminal residues of LCK and FYN in a dephosphorylated form that keeps basally active conformation of these proteins. A study by Dong et al. indicates that a substantial pool of LCK and FYN is active in naive CD4z T cells. TCR stimulated control cells showed an increase in the phosphorylation of LCK activating Tyr394. The authors have also shown that LAT depletion reduces FYN phosphorylation upon TCR stimulation by SEB. Thus LAT promotes TCR-induced phosphorylation of LCK and FYN tyrosines. Here we can show the  similarity between the existing experimental observations and the computed c-values. We have found lower c-value for LCK in an unAbMole Mepiroxol perturbed TCR signaling pathway. In contrast, it has become 0.91611 and 0.39188 in a pathogen perturbed system and in the system, where it is optimized for two conflicting AbMole Metyrapone objective functions, respectively. In the same way, we got lower c-value for FYN in an unperturbed TCR signaling pathway. In contrast, it has become 0.86331 and 0.76781 in a pathogen perturbed system and in the system, where it is optimized for two conflicting objective functions, respectively. Li et al. have investigated the activation of signaling molecules present at upstream of ERK and downstream of TCR in W97ALck expressing cells, and reported that the inability of W97ALck to support ERK activation was due to defect in the activation of Raf-1. One more study has reported that signaling ERK was dependent on the activation of Golgi membrane-associated N-Ras. Moreover, basal and stimulated levels of Erk-type MAPK phosphorylation were found to be sensitive to Mek1 inhibitor PD-98059 that indicates that the bacterial products activated the entire signaling cascade that coregulates IL-8 induction and secretion. We have also found lower c-value for RasGRP1 in an unperturbed TCR signaling pathway. In contrast, it has become 0.58313 and 0.51328 in a pathogen perturbed system and in the system, where it is optimized for two conflicting objective functions, respectively. Moreover, we have got lower c-value for MEK1/2 in an unperturbed TCR signaling pathway, respectively. In contrast, it has become 0.12904 and 0.33124 in a pathogen perturbed system and in the system, where it is optimized for two conflicting objective functions respectively. We have found lower cvalue for Raf in an unperturbed TCR signaling pathway. In contrast, it has become 0.49728 and 0.44493 in a pathogen perturbed system and in the system, where it is optimized for two conflicting objective functions, respectively.
similarity between the existing experimental observations and the computed c-values. We have found lower c-value for LCK in an unAbMole Mepiroxol perturbed TCR signaling pathway. In contrast, it has become 0.91611 and 0.39188 in a pathogen perturbed system and in the system, where it is optimized for two conflicting AbMole Metyrapone objective functions, respectively. In the same way, we got lower c-value for FYN in an unperturbed TCR signaling pathway. In contrast, it has become 0.86331 and 0.76781 in a pathogen perturbed system and in the system, where it is optimized for two conflicting objective functions, respectively. Li et al. have investigated the activation of signaling molecules present at upstream of ERK and downstream of TCR in W97ALck expressing cells, and reported that the inability of W97ALck to support ERK activation was due to defect in the activation of Raf-1. One more study has reported that signaling ERK was dependent on the activation of Golgi membrane-associated N-Ras. Moreover, basal and stimulated levels of Erk-type MAPK phosphorylation were found to be sensitive to Mek1 inhibitor PD-98059 that indicates that the bacterial products activated the entire signaling cascade that coregulates IL-8 induction and secretion. We have also found lower c-value for RasGRP1 in an unperturbed TCR signaling pathway. In contrast, it has become 0.58313 and 0.51328 in a pathogen perturbed system and in the system, where it is optimized for two conflicting objective functions, respectively. Moreover, we have got lower c-value for MEK1/2 in an unperturbed TCR signaling pathway, respectively. In contrast, it has become 0.12904 and 0.33124 in a pathogen perturbed system and in the system, where it is optimized for two conflicting objective functions respectively. We have found lower cvalue for Raf in an unperturbed TCR signaling pathway. In contrast, it has become 0.49728 and 0.44493 in a pathogen perturbed system and in the system, where it is optimized for two conflicting objective functions, respectively.
It binds with phosphorylated SLP76 functions as a scaffold bringing NCK and WASP into proximit
We have found higher c-value for Rho/Cdc42 in an unperturbed TCR signaling pathway. In contrast, it has become 0 and 0.31557 in a pathogen perturbed system and in the system, where it is optimized for two conflicting objective functions, respectively. Excessive interferon gamma release due to SEB has been linked with the development of a lifethreatening systemic inflammation. We can find its similarity with our results in the form of higher c-value for IFN-c in an infected TCR signaling pathway. It should be mentioned here that repeated injections of enterotoxin in mice resulted in high levels of IL-10, but there was also a dramatic decrease in all the other cytokines. A study by Ackermann et al. in human leukaemic mast cells demonstrated that SAg stimulation downregulates the production of IL-4. Thus, through these findings we can say that SAgs may also downregulate cytokine production. Similarly, Wang et al. has shown that CARMA1 functions as a scaffold protein to recruit PKC-h, Bcl10, and IKKb to the lipid rafts of the immunological synapse, which ultimately leads to the activation of NF-kB. A study by Narayan et al. showed that CARMA complex is required for the induction of NF-kB by AKT, along with PKC activation using a CARMA1-deficient T cell line. AKT has a role in NF-kB induction in T cells. It is here to be mentioned for information that AKT acts upstream of the IKK complex to increase IKK activation, IkB degradation and NF-kB nuclear entry. The AbMole Diperodon c-values for AKT1 was lower in an unperturbed TCR signaling pathway, whereas, it was higher in perturbed one and again lower in an integrated pathway which was optimized for two conflicting objective functions. The case is similar with IKK complex, where c-value is lower in an unperturbed TCR signaling pathway, which later has AbMole Folic acid increased  in a perturbed TCR signaling pathway. It has again decreased in an integrated pathway which was optimized for two conflicting objective functions. MALT1 is recruited to the lipid rafts of the immunological synapse following activation of TCR and CD28 coreceptor. This recruitment is dependent on CARMA1. MALT1, Bcl10, and CARMA1 form a trimolecular complex. Expression of a MALT1 deletion mutant has shown to completely block the CD3/CD28 costimulation-induced NF-kB activation. Considering the above experimental evidences for CARMA complex, we have also found lower c-value for CARMA complex in an unperturbed TCR signaling pathway. In contrast, it has become 0.81185 and 0.23229 in pathogen perturbed system and in the system, where it is optimized for two conflicting objective functions, respectively. NCK plays a pivotal role in TCR-induced reorganization of the actin cytoskeleton and the formation of the immunological synapse in T lymphocytes.
in a perturbed TCR signaling pathway. It has again decreased in an integrated pathway which was optimized for two conflicting objective functions. MALT1 is recruited to the lipid rafts of the immunological synapse following activation of TCR and CD28 coreceptor. This recruitment is dependent on CARMA1. MALT1, Bcl10, and CARMA1 form a trimolecular complex. Expression of a MALT1 deletion mutant has shown to completely block the CD3/CD28 costimulation-induced NF-kB activation. Considering the above experimental evidences for CARMA complex, we have also found lower c-value for CARMA complex in an unperturbed TCR signaling pathway. In contrast, it has become 0.81185 and 0.23229 in pathogen perturbed system and in the system, where it is optimized for two conflicting objective functions, respectively. NCK plays a pivotal role in TCR-induced reorganization of the actin cytoskeleton and the formation of the immunological synapse in T lymphocytes.
Retromer cargo proteins leading to various Wnt signaling related phenotypes
The promising results published in the present study should encourage exploring the detection of other pathogens with the same strategy. The mammalian intestinal epithelium is a rapidly selfrenewing tissue. Stem cells endow the intestine with its proliferative capacity. Intestinal stem cells reside at the bottom of  invaginations of the intestinal epithelium; the crypts of Lieberk��hn. The intestinal stem cells are characterized by expression of Lgr5, they are actively cycling and give rise to cells that proliferate in the transiently amplifying compartment of the crypt. Cells move up from the TA compartment and differentiate in the AbMole (R)-(-)-Modafinic acid villus domain. The villus epithelium consists of enterocytes, goblet cells and enteroendocrine cells. Paneth cells are differentiated cells that reside at the bottom of the crypt. The Paneth cells are part of the stem cell niche that supports the intestinal stem cells. Various signaling pathways- such as the Wnt, Notch and EGF signaling cascades are required to maintain intestinal homeostasis, but Wnt signaling is of particular importance because it drives proliferation and is essential for stem cell maintenance. Wnt signaling in intestinal stem cells is activated by Wnt ligands that are expressed in the Paneth cells and cells in the intestinal mesenchyme. Wnt signaling is enhanced by Rspondin, which is the ligand of the stem cell marker Lgr5. It is essential that a fine balance of Wnt pathway activity is AbMole Mepiroxol maintained in the intestine, as overactivation of Wnt signaling results in adenoma formation and ultimately leads to cancer. Detailed knowledge has accumulated about the mechanism of Wnt signal transduction in Wnt receiving cells, but the mechanism of Wnt secretion has only recently been uncovered. Wnt protein is produced in the ER and lipid modified by the O-acyltransferase Porcupine. Wnt follows the secretory pathway to the Golgi apparatus where it associates with Wntless, a transmembrane protein that is essential for Wnt secretion. Wls escorts Wnt from the Golgi to the plasma membrane where Wnt is released. Importantly, studies in C. elegans, Drosophila and mammalian tissue-culture cells have shown that Wls needs to be retrieved back to the trans-Golgi network to maintain Wnt secretion. This retrieval route involves AP-2 and clathrin mediated endocytosis of Wls from the plasma membrane and transport from endosomes to the TGN, a retrograde trafficking step that is mediated by the retromer complex. In the absence of a functional retromer complex, Wls is retained in the endosomal system and degraded in lysosomes. The retromer complex is a multi-protein complex that mediates transport of membrane proteins from endosomes to the TGN.
invaginations of the intestinal epithelium; the crypts of Lieberk��hn. The intestinal stem cells are characterized by expression of Lgr5, they are actively cycling and give rise to cells that proliferate in the transiently amplifying compartment of the crypt. Cells move up from the TA compartment and differentiate in the AbMole (R)-(-)-Modafinic acid villus domain. The villus epithelium consists of enterocytes, goblet cells and enteroendocrine cells. Paneth cells are differentiated cells that reside at the bottom of the crypt. The Paneth cells are part of the stem cell niche that supports the intestinal stem cells. Various signaling pathways- such as the Wnt, Notch and EGF signaling cascades are required to maintain intestinal homeostasis, but Wnt signaling is of particular importance because it drives proliferation and is essential for stem cell maintenance. Wnt signaling in intestinal stem cells is activated by Wnt ligands that are expressed in the Paneth cells and cells in the intestinal mesenchyme. Wnt signaling is enhanced by Rspondin, which is the ligand of the stem cell marker Lgr5. It is essential that a fine balance of Wnt pathway activity is AbMole Mepiroxol maintained in the intestine, as overactivation of Wnt signaling results in adenoma formation and ultimately leads to cancer. Detailed knowledge has accumulated about the mechanism of Wnt signal transduction in Wnt receiving cells, but the mechanism of Wnt secretion has only recently been uncovered. Wnt protein is produced in the ER and lipid modified by the O-acyltransferase Porcupine. Wnt follows the secretory pathway to the Golgi apparatus where it associates with Wntless, a transmembrane protein that is essential for Wnt secretion. Wls escorts Wnt from the Golgi to the plasma membrane where Wnt is released. Importantly, studies in C. elegans, Drosophila and mammalian tissue-culture cells have shown that Wls needs to be retrieved back to the trans-Golgi network to maintain Wnt secretion. This retrieval route involves AP-2 and clathrin mediated endocytosis of Wls from the plasma membrane and transport from endosomes to the TGN, a retrograde trafficking step that is mediated by the retromer complex. In the absence of a functional retromer complex, Wls is retained in the endosomal system and degraded in lysosomes. The retromer complex is a multi-protein complex that mediates transport of membrane proteins from endosomes to the TGN.