Although confirmation of the efficacy of this pharmacological approach awaits completion of large clinical trials, the adjuvant use of these compounds is common in patients that do not meet targeted reductions of lipoproteins while taking statins. Given the high prevalence of lipid metabolism disorders it is desirable to identify lead compounds that can be developed into new drugs that inhibit lipid absorption via novel mechanisms. Here we report the utility of using the zebrafish for this purpose. Because of their small size, optical transparency zebrafish larvae are well suited for chemical library screens using fluorescent, histochemical or morphological assays. Indeed, a great advantage of chemical screens in zebrafish is the ability to rapidly assess compound efficacy and toxicity in vivo. Given the high degree of conservation of lipid metabolism in teleost fish and mammals, it is likely that compounds identified in a zebrafish screen will act through comparable mechanisms in mammals. Here we report the results of a pilot screen of a non-biased chemical library through which we identified 7 novel compounds that inhibited the absorption of fluorescent lipid analogues. We show that compounds identified in the primary screening assay can be rapidly prioritized for testing in mammals using a variety of simple, yet Everolimus highly informative in vivo secondary assays. The secondary assays also Torin 1 company provided insights into the compounds�� mechanism of action, which could be 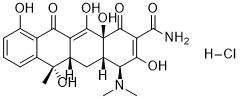 distinguished from the effects of orlistat and ezetimibe in zebrafish larvae. Surprisingly, we found that ezetimibe inhibited absorption of not only cholesterol analog, but also long chain fatty acid and phopholipid analogs. Together, these findings demonstrate the feasibility of conducting screens for compounds that interfere with complex physiological processes using the zebrafish. The screening assays used for this study were derived from previous work using fluorescent lipid reporters in zebrafish larvae. Following their ingestion, the fluorescent metabolites of these reporters are first detected in the gallbladder of live larvae and later the intestinal lumen following gallbladder contraction. The compounds are used at low concentrations and they are rapidly absorbed from the intestinal lumen, thus their fluorescence emission is not detected in the intestinal lumen immediately after ingestion or when absorption in inhibited. Fluorescence emission from one of the analogues, the phospholipid PED-6, is quenched prior to metabolism by luminal phospholipase. Thin layer chromatographic analyses of bile from adult fish, or total body lipids of 5 dpf larvae, showed that PED-6, which is labeled with a BODIPY labeled short chain fatty acid at the sn-2 position, is metabolized to cholesterol esters, phospholipids and possibly, triglyceride. Free PED6 was not detected in either assay. For the primary screen, 5 day post-fertilization larvae were arrayed in 96 well plates and soaked overnight in test compounds. The following morning larvae were soaked in PED-6 for 6 hours after which a qualitative visual assessment of gallbladder fluorescence was made using an inverted compound microscope. Reduced gallbladder fluorescence, the endpoint we use to identify active compounds in the primary screen, could not differentiate compounds that inhibited lipid absorption from those that interfered with swallowing, phospholipase activity or hepatic metabolism and biliary secretion. As described below, secondary assays were devised to distinguish these mechanistic possibilities.
distinguished from the effects of orlistat and ezetimibe in zebrafish larvae. Surprisingly, we found that ezetimibe inhibited absorption of not only cholesterol analog, but also long chain fatty acid and phopholipid analogs. Together, these findings demonstrate the feasibility of conducting screens for compounds that interfere with complex physiological processes using the zebrafish. The screening assays used for this study were derived from previous work using fluorescent lipid reporters in zebrafish larvae. Following their ingestion, the fluorescent metabolites of these reporters are first detected in the gallbladder of live larvae and later the intestinal lumen following gallbladder contraction. The compounds are used at low concentrations and they are rapidly absorbed from the intestinal lumen, thus their fluorescence emission is not detected in the intestinal lumen immediately after ingestion or when absorption in inhibited. Fluorescence emission from one of the analogues, the phospholipid PED-6, is quenched prior to metabolism by luminal phospholipase. Thin layer chromatographic analyses of bile from adult fish, or total body lipids of 5 dpf larvae, showed that PED-6, which is labeled with a BODIPY labeled short chain fatty acid at the sn-2 position, is metabolized to cholesterol esters, phospholipids and possibly, triglyceride. Free PED6 was not detected in either assay. For the primary screen, 5 day post-fertilization larvae were arrayed in 96 well plates and soaked overnight in test compounds. The following morning larvae were soaked in PED-6 for 6 hours after which a qualitative visual assessment of gallbladder fluorescence was made using an inverted compound microscope. Reduced gallbladder fluorescence, the endpoint we use to identify active compounds in the primary screen, could not differentiate compounds that inhibited lipid absorption from those that interfered with swallowing, phospholipase activity or hepatic metabolism and biliary secretion. As described below, secondary assays were devised to distinguish these mechanistic possibilities.
Therefore may be used as an adjuvant or alternative to HMG co-reductase inhibitors for the primary
Leave a reply