Fatty acid synthaseis the enzyme that is responsible for the cellular synthesis of palmitate. As a metabolic oncogene, FASN is constitutively overexpressed and hyperactive in aggressive breast carcinoma. The up-regulation of FASN in tumors is an early and nearly universal epigenetic change that is involved in the development, maintenance and enhancement of the malignant phenotype. We hypothesized that FASN was the key downstream MK-1775 effector of the bidirectional ER/HER2 crosstalk that promotes malignant phenotypes, such as proliferation, migration, apoptosis evasion and endocrine resistance, in ER+/HER2+ breast cancer cells. There is bidirectional crosstalk between FASN and HER2 in cancer cells.FASN overexpression positively correlates with HER2 amplificationin breast cancer cells. FASN is the downstream mediator of HER2 tumorigenicity and cancer progression. FASN inhibition decreases HER2 expression by upregulating PEA3, a HER2 transcriptional inhibitor, and by changing the lipid composition and function of tumor cell membranes, thereby altering the cellular localization of HER2. In addition, inhibiting FASN negatively affects the interaction between EGFR and HER2, which is a mechanism of trastuzumab resistance in breast cancer. FASN is regulated by estrogen in ER-positive breast cancer cells; estrogen 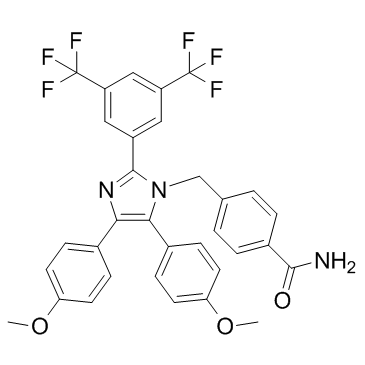 stimulates FASN expression. FASN expression is part of the E2-mediatedcellular response that leads to the proliferation of hormone-dependent carcinoma cells. Inhibiting FASN dramatically augments E2-stimulated, ER-driven transcriptional activity, synergistically enhances the E2-mediated down-regulation of ER expression and impairs E2-induced nuclear accumulation of ER. Furthermore, inhibiting FASN induces antitumor activity by acting as a SERM in ER-positive breast cancer cells.Therefore, FASN is most likely the downstream effector underlying ER/HER2 crosstalk in dual-positive breast cancer, but the signaling pathway that is involved remains unknown. The mammalian target of rapamycinsignaling pathway is one of the most important pathways in signal transduction in cancer. mTOR is a serine/threonine-specific kinase that is responsible for mitogen-induced cell proliferation, survival and motility in cancer cells. The mTOR signaling pathway may connect ER/HER2 crosstalk with the downstream effector FASN. HER2amplification activates the mTOR signaling pathway. Inhibiting mTOR blocks multiple stages of HER2induced tumorigenic progression and improves the antitumor activity of HER2 inhibitors.The mTOR pathway is also related to endocrine therapy resistance. Cycloheximide SERM-resistant MCF-7/ HER2 cells up-regulate mTOR expression by activating the PI3K/AKT, ERK and MAPK signaling pathways. The activated Phosphoinositide 3-kinase /AKT pathway stimulates mTOR to phosphorylate its downstream effectors p70 ribosomal S6 kinaseand eukaryotic initiation factor 4E binding protein 1, mediating the expression of genes associated with tumor malignancy. Therefore, the combination of mTOR inhibitors and hormone- or HER2-targeting therapies was believed to be a promising strategy for overcoming initial therapeutic resistance and for preventing the development of resistance in ER+/HER2+ breast cancer.There is an intimate relationship between FASN and mTOR. mTOR activation induces FASN expression, and inhibiting the mTOR signaling pathway down-regulates FASN expression. FASN inhibition upregulates DDIT4, a negative regulator of the mTOR pathway, suggesting that FASN inhibition negatively regulates the mTOR pathway via DDIT4. In this study, we found that FASN was overexpressed in ER+/ HER2+ breast cancer cells. And the transcriptional activity of FASN promoter was high in ER+/HER2+ breast cancer cells.
stimulates FASN expression. FASN expression is part of the E2-mediatedcellular response that leads to the proliferation of hormone-dependent carcinoma cells. Inhibiting FASN dramatically augments E2-stimulated, ER-driven transcriptional activity, synergistically enhances the E2-mediated down-regulation of ER expression and impairs E2-induced nuclear accumulation of ER. Furthermore, inhibiting FASN induces antitumor activity by acting as a SERM in ER-positive breast cancer cells.Therefore, FASN is most likely the downstream effector underlying ER/HER2 crosstalk in dual-positive breast cancer, but the signaling pathway that is involved remains unknown. The mammalian target of rapamycinsignaling pathway is one of the most important pathways in signal transduction in cancer. mTOR is a serine/threonine-specific kinase that is responsible for mitogen-induced cell proliferation, survival and motility in cancer cells. The mTOR signaling pathway may connect ER/HER2 crosstalk with the downstream effector FASN. HER2amplification activates the mTOR signaling pathway. Inhibiting mTOR blocks multiple stages of HER2induced tumorigenic progression and improves the antitumor activity of HER2 inhibitors.The mTOR pathway is also related to endocrine therapy resistance. Cycloheximide SERM-resistant MCF-7/ HER2 cells up-regulate mTOR expression by activating the PI3K/AKT, ERK and MAPK signaling pathways. The activated Phosphoinositide 3-kinase /AKT pathway stimulates mTOR to phosphorylate its downstream effectors p70 ribosomal S6 kinaseand eukaryotic initiation factor 4E binding protein 1, mediating the expression of genes associated with tumor malignancy. Therefore, the combination of mTOR inhibitors and hormone- or HER2-targeting therapies was believed to be a promising strategy for overcoming initial therapeutic resistance and for preventing the development of resistance in ER+/HER2+ breast cancer.There is an intimate relationship between FASN and mTOR. mTOR activation induces FASN expression, and inhibiting the mTOR signaling pathway down-regulates FASN expression. FASN inhibition upregulates DDIT4, a negative regulator of the mTOR pathway, suggesting that FASN inhibition negatively regulates the mTOR pathway via DDIT4. In this study, we found that FASN was overexpressed in ER+/ HER2+ breast cancer cells. And the transcriptional activity of FASN promoter was high in ER+/HER2+ breast cancer cells.
Involved in HER2 crosstalk may lead to the development of new strategies for the treatment of HER2
Leave a reply